Deinonychus: wolverine, ora, & eagle in one
Aug 15, 2022 22:55:33 GMT 5
Life, Grey, and 2 more like this
Post by Infinity Blade on Aug 15, 2022 22:55:33 GMT 5
© @ Matt Dempsey->
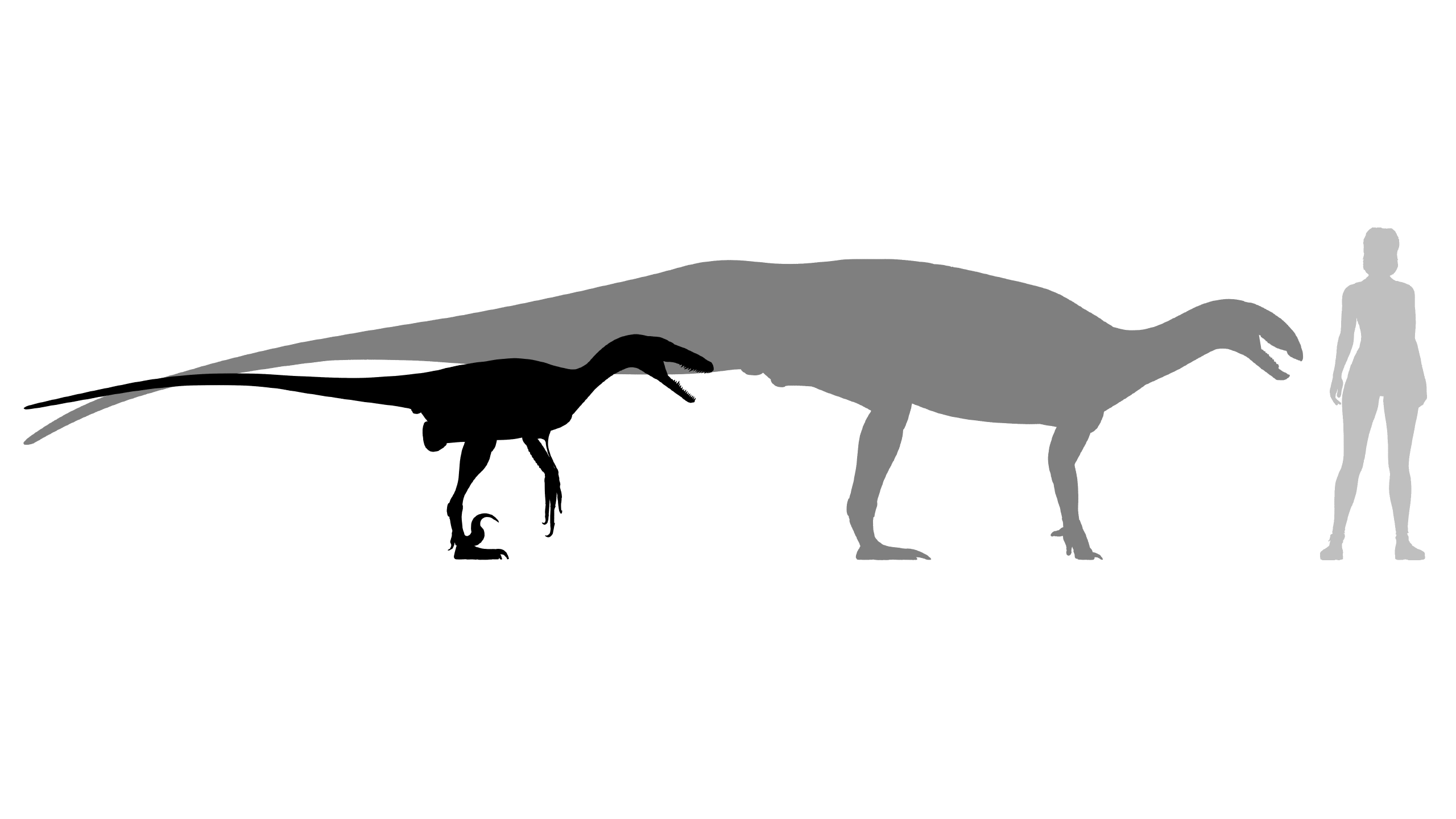
Deinonychus and Tenontosaurus have been associated with each other for decades as predator and prey. And this association is well-supported. Isotope analysis of adult Deinonychus teeth suggest it indeed fed on Tenontosaurus (Frederickson et al., 2020). Bite marks on Tenontosaurus bone by Deinonychus are also known (Gignac et al., 2010). But the former isotope study also argues against pack hunting (or at least mammal-like pack hunting, where the juveniles live with and feed on the same food as the adults) in Deinonychus. The default and most conservative assumption would be that Deinonychus was a solitary predator. The possibility of unrelated individuals opportunistically ganging up on large prey has not been ruled out by this study, but we also do not know how common such a practice was in D. antirrhopus. If we only assume the former, how could a single Deinonychus kill a Tenontosaurus?
The most obvious answer is targeting juveniles or immature individuals. Frederickson et al. (2020) find that their limited sample of juvenile Tenontosaurus had isotope signatures insignificantly different from their adult counterparts. It is likely, therefore, that Deinonychus often targeted young Tenontosaurus, although the limited sample size necessitates caution when interpreting these results. But even if you halved the size of an adult T. tilletti to assume an immature individual, the ornithopod would still be AT LEAST three, if not five times the size of the dromaeosaurid. Now, what about an adult Tenontosaurus?
Greg Paul’s most recent edition of the Princeton Field Guide to Dinosaurs puts D. antirrhopus at 60 kg, and T. tilletti at 600 kg (Paul, 2016). There are higher estimates of Deinonychus’ body mass (Paul, 1988; Campione et al., 2014), and T. dossi is estimated to have weighed even more than T. tilletti, at 1,000 kg (Paul, 2016). Therefore, it could be said that Tenontosaurus was roughly around ten times the size of Deinonychus. An adult, healthy Tenontosaurus was clearly a formidable prey item for Deinonychus. But predators killing prey around an order of magnitude larger than themselves are not unprecedented. There is at least one instance of a large leopard killing an adult bull eland (link->). Wolverines will jump onto the backs of reindeer, latch on with their forelimbs, and deliver nasty bites (Gishlick, 2008). Komodo dragons kill water buffalo up to twelve times their size. Golden eagles will kill domestic calves weighing as much as 230 kg, adult domestic sheep, and adult reindeer; they have been known to attack adult pronghorns and red deer (Mason, 2000). Here I argue how a Deinonychus could do the same with Tenontosaurus: by combining aspects from all of these predators into its modus operandi.
What Deinonychus takes from wolverines & big cats:
First and foremost, let’s establish this fact: Deinonychus had powerful forelimbs. The muscles used for forelimb adduction and flexion were especially strong, consistent with a raptorial function (Ostrom & Gauthier, 2019).


The claws on the fingers, of course, were recurved and sharp (link->).
These long, clawed forelimbs, therefore, would have been very useful for holding onto a large, struggling prey item. Previously the forelimbs were proposed to have been used for stability flapping in raptor prey restraint, but this is against small prey (Fowler et al., 2011). Large prey like a Tenontosaurus would need to be latched onto with the clawed forelimbs, lest the Deinonychus get bucked off. If, for some reason, you have any doubt that a Deinonychus would use its forelimbs for a grasping function, allow me to remind you that the Fighting Dinosaurs Velociraptor specimen literally has one of its forelimbs around the head of its Protoceratops quarry (link->).
Takeaway: the muscular, clawed forelimbs of Deinonychus would have been crucial for holding onto a large, struggling Tenontosaurus.
What Deinonychus takes from Komodo dragons:
Most people do not seem to understand the importance of the jaws in dromaeosaurid predation. This is no doubt due to the fame of their sickle claws. In this section I am going to stress this very important point: Deinonychus’ jaws were formidable weapons.
As was usually the case with carnivorous non-avian theropods, Deinonychus’ mouth was filled with ziphodont dentition. These teeth are recurved backwards, lateromedially compressed, and outfitted with serrated cutting edges on both the front and back surfaces. This gives the teeth a form and function akin to a steak knife.
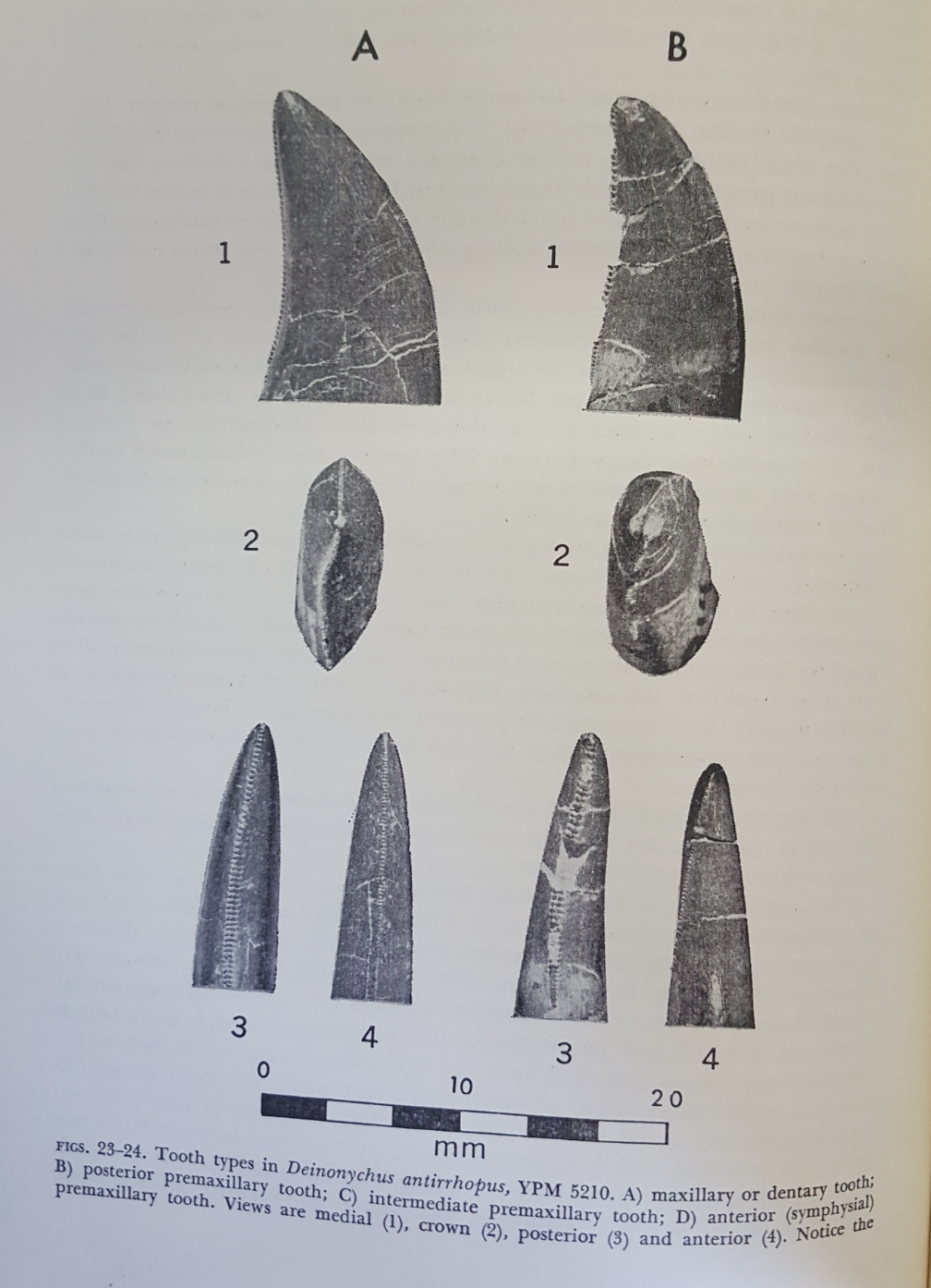
Deinonychus teeth.
This is the very same kind of dentition seen in the mouth of the modern Komodo dragon.

Komodo dragon tooth.
It’s clear that the teeth of Deinonychus would function in very much the same way that the teeth of a Komodo dragon would. Of course, Deinonychus did not have anti-coagulant venom (which the Komodo dragon may or may not actually use for predation). Nevertheless, these teeth would be superb at slicing through skin, flesh, blood vessels, and soft tissue in general. In fact, one of the references I cited in the very beginning of this post documents deep bite marks in the bone of a Tenontosaurus. This bite mark would have required 4,100 N behind it in order to cause the damage that it did (Gignac et al., 2010).

Yet the skull of Deinonychus was not suited for biting down particularly hard, and other studies have failed to replicate this same level of bite force for the species (Therrien et al., 2005; Sakamoto, 2022). How do we reconcile these two facts together?
The answer is simple. Deinonychus wasn’t using its jaw adductors to produce this kind of force. It was using other muscles in its body, like the whole head and neck system, to do so (Sakamoto, 2022).
It is important to note that forces necessary to puncture substrate are not confined to muscle-driven biting and may very well be the product of more aggressive kinetic feeding behaviours involving the whole head and neck (D’Amore & Blumenschine, 2009; Snively et al., 2013; Torices et al., 2018). This is supported by the observation that this bite mark in question was matched to a premaxillary tooth (Gignac et al., 2010), meaning that a long-snouted animal would have had to be capable of generating FBite of 3,000–4,000N (Gignac et al., 2010) at the tip of its snout through muscle generated biting, which is not congruent with the available data in comparably-sized amniotes (Sakamoto, Ruta & Venditti, 2019).
Does this sound familiar (D'Amore et al., 2011)?
What did Deinonychus’ skull look like? Was Deinonychus’ skull really suited for hunting large prey? The answer is…yes. Recent, though not yet properly published, research has found that Deinonychus actually had a short, stout snout (previous reconstructions have failed to account for post-mortem taphonomy on Deinonychus skull material). In this respect, dromaeosaurids are convergent with canids in how snout morphology is correlated with prey preference (shorter, more robust snouts are for large prey, while longer, narrower snouts are for small prey).
The snout of Deinonychus antirrhopus may be narrow relative to larger theropods, but the development of the anteromedial process of the maxilla and the lateral expansion of the nasals posteriorly clearly demonstrate there was a broad snout relative to other dromaeosaurids (Fig. 2.13C-D). The nasals are unknown for Atrociraptor marshalli but based on all the other proportions, this taxon appears to have converged on the short snout morphology of Deinonychus antirrhopus. This pronounced stoutness of the snouts in these taxa may reflect an ecomorphological adaptation for specialization on larger prey (Slater et al. 2009).
Additionally, these two taxa share dentitions of posteriorly angled (‘raked’) maxillary teeth (Character 83, Currie and Evans 2019). The re-curvature of teeth have been shown to increase posteriorly along the tooth row in theropods (D’Amore 2009). In the case of a short maxilla, it is possible that the ‘raking’ (inclination towards the throat) of the maxillary teeth relates to the line of action required for teeth during biting. The juvenile specimen of Bambiraptor feinbergi (AMNH FARB 30556) also has raked maxillary teeth and shares similar maxillary proportions to Atrociraptor marshalli and Deinonychus antirrhopus (Table 2.1). The condition is well contrasted by the tooth orientation in the relatively long snouted eudromaeosaurians Tsaagan mangas and Velociraptor sp., which have teeth that are oriented more perpendicular to the maxilla, although the most anterior teeth have slight anterior orientations. Contrasting tooth orientation supports the possibility that the abbreviation of the snout anteroposteriorly in Atrociraptor marshalli and Deinonychus antirrhopus serves a functional purpose for prey capture and handling. These two dromaeosaurids were separated by a large amount of time but converged on morphologies shown to be conducive to handling larger prey for modern carnivores (Slater et al. 2009).
Additionally, these two taxa share dentitions of posteriorly angled (‘raked’) maxillary teeth (Character 83, Currie and Evans 2019). The re-curvature of teeth have been shown to increase posteriorly along the tooth row in theropods (D’Amore 2009). In the case of a short maxilla, it is possible that the ‘raking’ (inclination towards the throat) of the maxillary teeth relates to the line of action required for teeth during biting. The juvenile specimen of Bambiraptor feinbergi (AMNH FARB 30556) also has raked maxillary teeth and shares similar maxillary proportions to Atrociraptor marshalli and Deinonychus antirrhopus (Table 2.1). The condition is well contrasted by the tooth orientation in the relatively long snouted eudromaeosaurians Tsaagan mangas and Velociraptor sp., which have teeth that are oriented more perpendicular to the maxilla, although the most anterior teeth have slight anterior orientations. Contrasting tooth orientation supports the possibility that the abbreviation of the snout anteroposteriorly in Atrociraptor marshalli and Deinonychus antirrhopus serves a functional purpose for prey capture and handling. These two dromaeosaurids were separated by a large amount of time but converged on morphologies shown to be conducive to handling larger prey for modern carnivores (Slater et al. 2009).
The effects of post-mortem deformation on the interpretations of morphology can be quite extensive as exemplified by the maxillae of Acheroraptor temertyorum and Deinonychus antirrhopus. The initial interpretation of the latter has led to drastically different interpretations of the skull shape (Ostrom 1969) that can have serious implications for understanding the ecology and phylogeny of the animal. The interpretation put forth in this study presents a reconstruction of Deinonychus antirrhopus that is much more similar to the general morphologies of other dromaeosaurids (Fig. 2.15) (Currie 1995, Burnham et al. 2000, Norell et al. 2006, Xu et al. 2010a, Currie and Evans 2019). It also reveals features of the maxilla not previously described, such as the anteromedial process, which was initially described as a premaxillary process of the nasal (Ostrom 1969). With the re-examination of YPM 5253 (557) it has also been shown that the anterior ramus is not elongate compared to the maxilla (Character 28, Currie and Evans 2019), nor does it possess an elongate shape (length to height ratio of 1 or greater) as in other eudromaeosaurians, even though it has been coded as such in recent phylogenetic analyses (Longrich and Currie 2009, Evans et al. 2013, Currie and Evans 2019). This removes some support for the close relationship of Deinonychus antirrhopus to velociraptorines and demonstrates that Deinonychus antirrhopus was more like North American forms like Atrociraptor marshalli, Bambiraptor feinbergi, Saurornitholestes langstoni. However, it is most similar to the Early Cretaceous Achillobator giganticus regarding the shape and length of the anterior ramus.

Skull reconstruction of Deinonychus antirrhopus.
era.library.ualberta.ca/items/c9c289c0-7cc4-42a0-ac53-9ffebf111d16/view/3c913f54-988b-40b8-8db2-f7ad9dee46e5/Powers_Mark_J_202006_MSc.pdf
A final note: Deinonychus skulls could actually reach 41 cm in length (Paul, 1988). That is much larger than any Komodo dragon skull. Consequently, Deinonychus could a) bite much larger, wider surfaces and b) take out much bigger bites out of its prey than a Komodo dragon could.
Takeaway: Deinonychus’ skull would have been crucial for killing large prey. Its massive skull filled with flesh-slicing, bone-puncturing teeth would have been used to rip out large pieces of flesh and induce severe blood loss. The whole head and neck, as opposed to the jaw adductors, would have provided the bulk of the force behind the teeth.
What Deinonychus takes from eagles:
Of course, no discussion about dromaeosaurid killing methods would be complete without mentioning the sickle claws on their feet. One important thing to remember is that Deinonychus (and by extension, other dromaeosaurids) could flex the toes of their feet in a way reminiscent of a clenched fist (Fowler et al., 2011; Fig. 8).

The most obvious function of these sickle claws, therefore, is to maintain a tight grip on the struggling Tenontosaurus. One recent biomechanical study found that the amount of force at the tip of the sickle claw was maximized when the hindlimb was in a crouched position (Bishop, 2019). Granted, this study only mapped two muscles, but if the results are to be taken at face value, then it has some interesting implications for the matter discussed here. When a Deinonychus is latched onto the body of a Tenontosaurus at close range, its hindlimbs would be in a flexed, crouched position. This would be ideal for force production at the tip of the claw.
Does this mean Deinonychus’ toe claw was not a lethal weapon, but just a gripping tool? Not at all. The talons of modern eagles can produce very severe wounds when riding on large prey.

The more a Tenontosaurus struggles, the deeper the talons of a Deinonychus will sink into the flesh of its victim. Likewise, as the raptor is trying to wrench off pieces of flesh with its mouth, one could imagine the Deinonychus pushing its feet against the body of the Tenontosaurus to brace itself. That, too, will sink the claws further in. This could produce severe puncture wounds or even lacerations. If a Deinonychus really wanted to, I suppose it could try raking/slashing with its claws like the stereotypical image of dromaeosaurids, although, it might be in its best interest to try to hold on with its clawed feet (and let the violent struggling do all the work).
Takeaway: the clawed feet of Deinonychus were crucial for gripping onto a large struggling prey item, as well as producing life-threatening punctures and lacerations.
Conclusion:
Taken together, all this information points towards Deinonychus, and dromaeosaurids in general, functioning like a well-oiled machine when tackling prey multiple times larger than themselves. The forelimbs gripped as hard as they could (although, some raking for extra blood loss is possible). The same could be said of the hindlimbs, although the wounds they would have ended up producing would have been horrific. The jaws were completely dedicated to causing damage and bleeding the prey out.
While there were certainly plenty of small prey items for Deinonychus to hunt, prey multiple times its size, like Tenontosaurus, were not out of the question. It would essentially have hunted and killed creatures like the giant ornithopod like a wolverine attacking a reindeer. Except it uses the mouth and teeth of a Komodo dragon to bite with, and the talons of an eagle to additionally grip, stab, or even cut with.