Post by Life on Aug 6, 2010 13:29:53 GMT 5
The Ginsu Shark (Cretoxyrhina mantelli)

Credit: Fossil Crates (modified; depiction with battle scars)
Classification
[1] Information sourced from Amalfitano et al (2019).
[2] Information sourced from Diedrich (2014).
---
Overview from Fossil Crates
-----
Fossil record
Members of the genus Cretoxyrhina including Cretoxyrhina mantelli are among the best preserved sharks in the world.
Figure 4 from Diedrich (2014) [2] reference:
 [2]
[2]
CAPTION: Interpretative drawings of Late Cretaceous Isurus-skeletons. (a) Partly disarticulated skeleton without skull from the late Turonian of Germany (NMB no. 3916 Foerth-Isurus-1); (b) partly disarticulated but nearly complete skeleton from the Niobrara Formation (Coniancian to Campanian) of USA with its last prey, a huge teleostean Xiphactinus audax (in black, redrawn after [18]; KUVP no. 247 after [19]); (c) articulated skeleton from the Niobrara Formation (Coniancian to Campanian) of USA (redrawn after [13]; FHSM no. VP-2187); (d) partly preserved articulated skeleton from the “Senonian” (Coniacian/Santonian) of Italy (redrawn after [20]); (e) partly preserved articulated skeleton from the Niobrara Formation (Coniancian to Campanian) of USA (redrawn after photos of Everhart, STERN no. 01).
NOTE: Diedrich (2014) [2] is/was a proponent of the taxonomic re-assignment of Cretoxyrhina mantelli to Isurus denticulatus much like Glikman (1958) but this re-assignment is in dispute and/or rejected:
The best preserved specimens on record are following:
---
Largest teeth ever found
Figure S2 in Pimiento et al (2010) [5] for reference:
 [5]
[5]
FFHM 1972.196 as depicted in Shimada (2008) [4] for reference:
 [4]
[4]
CAPTION: FIGURE 6. Anterior teeth of Cretoxyrhina mantelli from Niobrara Chalk of western Kansas. A, shortest known anterior tooth (FHSM VP- 16522); B, tallest known anterior tooth (FFHM 1972.196; tooth tip re-stored). Left = labial view; right = lingual view.
FFHM 1972.196 in restored form for reference:
 [6]
[6]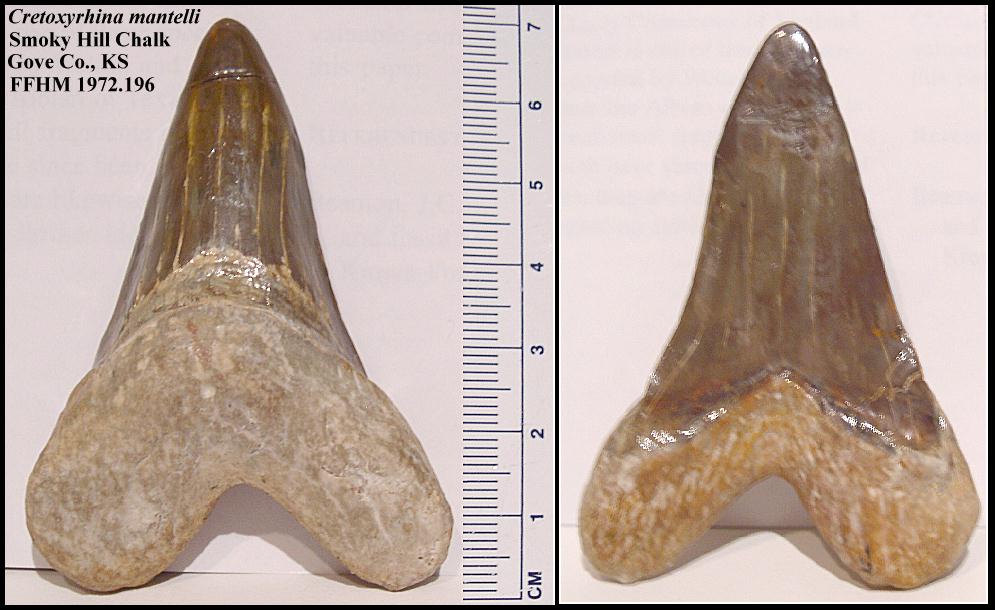
---
Largest vertebral centrum ever found
The largest vertebral centrum preserved is of the Cretoxyrhina mantelli specimen MPPSA-IGVR 36371; it has a diameter of 107 mm (Amalfitano et al., 2019) [1]; not shown in the photo below.
 [1]
[1]
CAPTION: Fig. 15. Cretoxyrhina mantelli (Agassiz, 1835). Count of incremental bands of individuals MPPSA-IGVR 36371 and 45305. A. Vertebral centrum from MPPSA-IGVR 36371. The black dot indicates the vertebral fulcrum, the black stars indicate the incremental bands (26). B. Vertebral centrum from MPPSA-IGVR 45305. The black dot indicates the vertebral fulcrum, the black stars indicate the incremental bands (21). Scale bars = 10 mm [1,5-column width].
---
TL estimations
Amalfitano et al (2019) [1] provide an overview:
-----
Physiology and anatomy
 [7]
[7]
Description: 25-foot Cretaceous era shark Cretoxyrhina. McWane Science Center, Birmingham, AL. Photo credit: John Gnida.
---
Dentition and jaw structure
 [8]
[8]
NOTE: This partially preserved dentition is in possession of Dr. Gordon Hubbell (private collection).
---
Partially preserved dentition (specimen FHSM VP-14004) in Bourdon and Everhart (2011) [9] for reference:
 [9]
[9]
CAPTION: FACING PAGE - Figure 6, Cretoxyrhina mantelli, FSHM VP-14004 tooth set and examples, individually scaled by group A-F, Tooth set, 3-perspectives, similarly scaled. Scale bar = 3 cm; A, Upper files, basal view; B, Upper files, labial view; C, Lower files labial view; D, Lower files, basal view; E, Upper files, lateral view; F, Lower files, lateral view;
---
Dental pattern in Bourdon and Everhart (2011) [9] for reference:
 [9]
[9]
CAPTION: Figure 2, Cretoxyrhina tooth set (FHSM VP-2187) with Comparative Position notations. Tooth set source Shimada (1994), reproduced with permission.
A closer look at specimen FHSM VP-2187 for reference:

 [6]
[6]
A closer look at the skull of specimen FHSM VP-2187 for reference:
 [10]
[10]
Reconstructed skull in Shimada (1997) [11] for reference:
 [11]
[11]
A closer look at the skull of 25-foot Cretaceous era shark Cretoxyrhina in McWane Science Center, Birmingham, AL:
 [12]
[12]
---
Body structure
Figure 1 in Shimada (2008) [4] for reference:
 [4]
[4]
CAPTION: FIGURE 1. Late Cretaceous lamniform shark, Cretoxyrhina mantelli. A, nearly complete skeleton, FHSM VP-2187, from Niobrara Chalk, Ellis County, Kansas (see Shimada, 1997c; vertebra immediately below asterisk is vertebra illustrated in Figure 2); B, skeletal reconstruction based on multiple skeletal remains (after Shimada, Cumbaa et al., 2006: fig. 8; maximum total length = approximately 7 m in total length; solid lines = known parts; broken lines = inferential parts).
---
Fin structure
Partially preserved pectoral fin of specimen KUVP 49490 in Shimada (2008) [4] for reference:
 [4]
[4]
CAPTION: FIGURE 7. Pectoral fin radials (KUVP 49490). with an outline of the entire fin. of Cretoxyrhina(?) (anterior to the top).
Partially preserved caudal fin of specimen CMN 40906 in Shimada et al (2006) [13] for reference:
 [13]
[13]
CAPTION: FIGURE 4. Close-up view of putative caudal fin base, showing connection between vertebral column and hypochordal rays in CMN 40906 (scale bar = 10 cm; cf. Figs. 1,2).
---
Regional endothermy
Ferrón (2017) [14] present a well-researched case for regional endothermy in Cretoxyrhinids; see REFERENCES for access to full article.
Figure 4 in Ferrón (2017) [14] for reference:
 [14]
[14]
CAPTION: Fig 4.
(A) Comparison between net cost of swimming (NCS) and routine metabolic rate (RMR) of Cretoxyrhina mantelli at five different temperature scenarios (5°C, 10°C, 15°C, 20°C and 25°C) assuming ectothermy (green) and regional endothermy (pink). Green and pink gradations represent the NCS at different swimming speeds (see color code chart). Note that NCS are constant in all temperature scenarios (see text). RMR estimate is represented with associated 95% individual prediction intervals (in black). (B) Validation test performed in 17 living sharks, including ectothermic taxa (green background) and regional endothermic taxa (pink background), from simultaneous records of their cruise swimming speeds, water temperatures and body masses (data taken from [85]). Inferred RMR are denoted as green and pink intervals for the ectothermic and regional endothermic scenario respectively; Inferred NCS are represented by black dots.
LINK: doi.org/10.1371/journal.pone.0185185.g004
---
Speed and swimming behavior
Cretoxyrhina mantelli was a fast-moving shark with estimated burst swimming capacity of 30.55 km/h.
Figure 2 in Ferrón (2017) [14] for reference:
 [13]
[13]
CAPTION: Fig 2. Scaling of swimming speed in extant fishes and swimming speed inferences in cretoxyrhinids and otodontids.
(A) Cruise and (B) burst relative swimming speeds (U, body lengths*s-1) against body lengths (meters) of living ectothermic and regional endothermic fishes. Adjusted regression lines are showed with associated 95% confidence intervals. (C) Cruise and (D) burst swimming speed estimates (V, km*h-1) of cretoxyrhinids and otodontids, considering them as ectothermic sharks (green) or regional endothermic sharks (pink), with associated 95% individual prediction intervals. Values of absolute (V, km*h-1) and relative (U, BL*s-1) speed estimates are also shown for each case. ** indicates significance at the 0.05 level.
LINK: doi.org/10.1371/journal.pone.0185185.g002
Pointer # 1 in relation:
NOTE: see Trophic interactions section below.
Pointer # 2 in relation:
Traces of scales on specimen FHSM VP-2187 in Shimda (1997) [11] for reference:
 [11]
[11]
NOTE: Two related Figures depicted side-by-side for a better view.
Traces of scales on specimen specimen CMN 40906 in Shimada et al (2006) [13] for reference:
 [13]
[13]
NOTE: Two related Figures depicted side-by-side for a better view.
Amalfitano et al (2019) [1] provide further insight in relation:
---
Longevity
Cretoxyrhina mantelli experienced intense competitive pressures from other contemporaneous macrophagous taxons (see Overview from Fossil Crates section above), and chances of mortality were high in the neonate stage in particular (Shimada, 2008) [4].
Shimada (2008) [4] asserted that Cretoxyrhina mantelli could exceed 30 years in life:
 [4]
[4]
- but this inference is not independently cross-validated in the present.
Cretoxyrhina mantelli had estimated TL of 1.27 m at birth (Shimada. 2008) [4]; give or take:
 [4]
[4]
Figure 2 in Shimada (2008) [4] for reference:
 [4]
[4]
CAPTION: FIGURE 2. Vertebral centrum of Cretoxyrhina mantelli (FHSM VP- 2187) showing diagenetically stained zones interpreted to be growth increment bands on cross-sectional surface of corpus calcareum (after Shimada, 1997a: fig. 1). In this illustration, band count is 15, not including band formed presumably at birth (i.e., band number 0). Arrow points the center of vertebra.
-----
Trophic interactions
Competitive pressures notwithstanding, Cretoxyrhina mantelli was built to kill:
Shimada (1997) [15] highlighted the possibility of Cretoxyrhina mantelli being the apex predator of its time:
There is ample fossil record of trophic interaction(s) of prehistoric sharks [3][15]. Examples of stomach contents and/or cases of trophic interactions of Cretoxyrhina mantelli are presented below:
a misidentified as Squalicorax in Schwimmer et al (1997); revisited in (Shimada & HOOKS III., 2004) [17]
b misidentified as Squalicorax in Martin and Rothschild (1989); revisited in (Shimada., 1997) [15]; revisit acknowledged in (Rothschild et al., 2005) [18]
c this specimen could be a member of genus Tylosaurus in life but correct identification is not possible due to condition of the remains (Rothschild et al., 2005) [18]
d these teeth are identified as FHSM VP-13284 and FHSM VP-13285 respectively (Shimada., 1997) [15]
e this specimen show signs of recovery [6], but this inference is disputed in (Shimada, 1997) [15]; attack on the skull or head region is typically indicative of predation effort and/or aggressive trophic interaction (Everhart, 2008; Voss et al., 2019; Bastiaans et al., 2020) [20][21][22]
f estimate(s) based on relevant information in Everhart (2002) [23]
---
Rothschild et al (2005) [18] disclose that the angle of approach towards Mosasaurs varied:
Following image is helpful in this regard:
 [6]
[6]
Related information in the following link of [6]: oceansofkansas.com/bite.html
Rothschild et al (2005) [18] noted that Cretoxyrhina mantelli was bigger than any mosasaur at TL parity:
---
Summary of Shimada (1997) [15] for reference:
 [15]
[15]
Rothschild et al (2005) [18] noticed and examined several cases of trophic interactions of prehistoric sharks with Mosasaurs and asserted that the survival rate of Mosasaurs was much better in trophic interactions with sharks in the (TL = 2 - 3 m) range but attacks from much larger sharks were most likely FATAL:
NOTE: Mosasaurs approaching 7 m in TL are much larger in comparison to those around 5 m in TL as apparent in Figure 6 of Everhart (2008) [20] below:
 [20]
[20]
CAPTION: Figure 6. Relative sizes of the two mosasaurs (7 m and 5 m). Scale = 1 m. (Adapted from Williston, 1898)
Everhart (2008) [20] disclosed a case of trophic interaction between a Mosasaur (TL = 7 m) and another Mosasaur (TL = 5 m) in fact; the larger Mosasaur aimed for the skull of the other Mosasaur and crushed it. Mosasaurs exceeding 5 m in TL were adults of some species (Everhart, 2002; Everhart, 2008) [23][20].
Additional evidence of trophic interactions with Mosasaurs
Related information in following link of [6]: oceansofkansas.com/mosapath.html
There is additional evidence of the fact that Mosasaurs were VULNERABLE to trophic interactions with contemporaneous sharks. This dynamic is most apparent in trophic interaction(s) of the Tylosaurus specimen FHSM VP-13750 with two sharks at different points in time.
+
Tylosaurus specimen FHSM VP-13750 contain a shark tooth driven into it:
 [24]
[24]
CAPTION: Fused caudal vertebrae at the end of the tail of a “club tail” Tylosaurus (FHSM VP-13750) in left lateral view (upper) and right lateral view (lower). Anterior is to the left. The embedded tooth of a shark, the apparent cause of the infection, is located within the white oval. After healing, the fused mass and 28 associated caudal vertebrae of this specimen were subsequently consumed and partially digested by another predator. Scale bar = 2 cm.
Closer look:

 [6]
[6]
This Mosasaur was a juvenile at the time of the afore-depicted instance of trophic interaction with a shark (trophic interaction # 1) and recovered:
This Mosasaur endured the FIRST attack (trophic interaction # 1) but suffered mobility degradation and could not endure SECOND attack (trophic interaction # 2):
The another predator in the case of specimen FHSM VP-13750 is believed to be a shark as well:
Full view of Tylosaurus specimen FHSM VP-13750 for reference:
 [6]
[6]
Description: At first glance, this pile of mosasaur caudal vertebrae (FHSM VP-13750) appeared to be evidence of just another mosasaur tail bitten off by a large shark and partially digested before being regurgitated. However, the odd shaped lump of bone in the lower left turns out to be at least 3 and probably 4 caudal vertebrae that have been fused together as the result of an infection. It appears likely that the mosasaur had the end of it's tail bitten off by an unknown predator (probably a shark). The wound became infected, fusing the vertebrae and more or less forming a bony club at the end of the tail as it healed. The mosasaur survived the initial attack but then was "sliced and diced" some time afterwards by a large shark, most likely Cretoxyrhina [6].
-----
Evolution and timeline of existence
Cretoxyrhina mantelli had a cosmopolitan distribution, and the current impression is that it might be a chronospecies:
Geological timescale for reference:
 [25]
[25]
Rothschild et al (2005) [18] suggest that Cretoxyrhina mantelli suffered extinction by the onset of the Late Campanian:
Lindgren (2004) [29] highlight a near-extinction event near the Mid-Campanian:
Ikejiri and Zhang (2020) [30] provide further insight in relation:
It is possible that near- and mid- Campanian disruptions created 'sustainability crisis' for Cretoxyrhina mantelli and contributed to its decline and subsequent extinction on a wider level.
-----
REFERENCES
[1] Amalfitano, J., Giusberti, L., Fornaciari, E., Dalla Vecchia, F. M., Luciani, V., Kriwet, J., & Carnevale, G. (2019). Large deadfalls of the ʻginsuʼ shark Cretoxyrhina mantelli (Agassiz, 1835)(Neoselachii, Lamniformes) from the Upper Cretaceous of northeastern Italy. Cretaceous Research, 98, 250-275.
[2] Diedrich, C. G. (2014). Skeleton of the fossil shark Isurus denticulatus from the Turonian (Late Cretaceous) of Germany—ecological coevolution with prey of mackerel sharks. Paleontology Journal, 2014.
[3] Everhart, M. J. (2005). Bite marks on an elasmosaur (Sauropterygia; Plesiosauria) paddle from the Niobrara Chalk (Upper Cretaceous) as probable evidence of feeding by the lamniform shark, Cretoxyrhina mantelli. PalArch, Vertebrate Paleontology, 2(2), 14-24.
[4] Shimada, K. (2008). Ontogenetic parameters and life history strategies of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli, based on vertebral growth increments. Journal of Vertebrate Paleontology, 28(1), 21-33.
[5] Pimiento, C., Ehret, D. J., MacFadden, B. J., & Hubbell, G. (2010). Ancient nursery area for the extinct giant shark Megalodon from the Miocene of Panama. PLoS one, 5(5), e10552.
[6] Link: oceansofkansas.com/KS-sharks.html
[7] Link: allosaurusroar.com/mcwane-science-center-birmingham-al/
[8] Link: tellmewhereonearth.com/Sharks_Page_1.htm
[9] Bourdon, J., & Everhart, M. J. (2011). Analysis of an associated Cretoxyrhina mantelli dentition from the Late Cretaceous (Smoky Hill Chalk, Late Coniacian) of western Kansas. Transactions of the Kansas Academy of Science, 114(2), 15-32.
[10] Link: sternbergca.fhsu.edu/index.php/Detail/objects/74c6eaaa-30cb-4327-9877-5d8611c95aea#
[11] Shimada, K. (1997). Skeletal anatomy of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli from the Niobrara Chalk in Kansas. Journal of Vertebrate Paleontology, 17(4), 642-652.
[12] Link: www.bonhams.com/auctions/17517/lot/37/?category=list
[13] Shimada, K., Cumbaa, S. L., & Rooyen D. V. (2006). Caudal fin skeleton of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli, from the Niobrara Chalk of Kansas. New Mexico Museum of Natural History and Science Bulletin, 35, 185-192.
[14] Ferrón, H. G. (2017). Regional endothermy as a trigger for gigantism in some extinct macropredatory sharks. PLoS one, 12(9), e0185185.
[15] Shimada, K. (1997). Paleoecological relationships of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli (Agassiz). Journal of Paleontology, 926-933.
[16] Shimada, K., & Everhart, M. J. (2004). Shark-bitten Xiphactinus audax (Teleostei: Ichthyodectiformes) from the Niobrara Chalk (Upper Cretaceous) of Kansas. The Mosasaur, 7, 35-39.
[17] Shimada, K., & HOOKS III, G. E. (2004). Shark-bitten protostegid turtles from the Upper Cretaceous Mooreville Chalk, Alabama. Journal of Paleontology, 78(1), 205-210.
[18] Rothschild, B. M., Martin, L. D., & Schulp, A. S. (2005). Sharks eating mosasaurs, dead or alive?. Netherlands Journal of Geosciences, 84(3), 335-340.
[19] Everhart, M. J. (2005). Tylosaurus kansasensis, a new species of tylosaurine (Squamata, Mosasauridae) from the Niobrara Chalk of western Kansas, USA. Netherlands Journal of Geosciences, 84(3), 231-240.
[20] Everhart, M. J. (2008). A bitten skull of Tylosaurus kansasensis (Squamata: Mosasauridae) and a review of mosasaur-on-mosasaur pathology in the fossil record. Transactions of the Kansas Academy of Science, 111(3), 251-262.
[21] Voss, M., Antar, M. S. M., Zalmout, I. S., & Gingerich, P. D. (2019). Stomach contents of the archaeocete Basilosaurus isis: Apex predator in oceans of the late Eocene. PLoS one, 14(1), e0209021.
[22] Bastiaans, D., Kroll, J. J., Cornelissen, D., Jagt, J. W., & Schulp, A. S. (2020). Cranial palaeopathologies in a Late Cretaceous mosasaur from the Netherlands. Cretaceous Research, 112, 104425.
[23] Everhart, M. J. (2002). New data on cranial measurements and body length of the mosasaur, Tylosaurus nepaeolicus (Squamata; Mosasauridae), from the Niobrara Formation of western Kansas. Transactions of the Kansas Academy of Science, 105(1), 33-43.
[24] Rothschild, B., & Everhart, M. J. (2015). Co-ossification of vertebrae in mosasaurs (Squamata, Mosasauridae); Evidence of habitat interactions and susceptibility to bone disease. Transactions of the Kansas Academy of Science, 118(3-4), 265-275.
[25] Adopted from: www.geosociety.org/GSA/Education_Careers/Geologic_Time_Scale/GSA/timescale/home.aspx
[29] Lindgren, J. (2004). Stratigraphical distribution of Campanian and Maastrichtian mosasaurs in Sweden–evidence of an intercontinental marine extinction event?. GFF, 126(2), 221-229.
[30] Ikejiri, T., Lu, Y., & Zhang, B. (2020). Two-step extinction of Late Cretaceous marine vertebrates in northern Gulf of Mexico prolonged biodiversity loss prior to the Chicxulub impact. Scientific reports, 10(1), 1-13.

Credit: Fossil Crates (modified; depiction with battle scars)
Classification
| Kingdom | Animalia | ||
| Phylum | Chordata | ||
| Subphylum | Vertebrata | ||
| Class | Chondrichthyes | Huxley, 1880 | [1] |
| Subclass | Elasmobranchii | Bonaparte, 1838 | [1] |
| Cohort | Euselachii | Hay, 1902 | [1] |
| Subcohort | Neoselachii | Compagno, 1977 | [1] |
| Superorder | Galeomorphii | Compagno, 1973 | [1] |
| Order | Lamniformes | Berg, 1937 | [1] |
| Family | Cretoxyrhinidae | Glikman, 1958 | [1] |
| Genus | Cretoxyrhina* | Glikman, 1958 | [1] |
| Species | Cretoxyrhina mantelli (also identified as Oxyrhina mantelli [1] and Isurus denticulatus) [1][2] | Agassiz, 1835 | [1] |
*This genus have following documented species:
| Cretoxyrhina vraconensis | [1] |
| Cretoxyrhina agassizensis | [1] |
| Cretoxyrhina denticulata | [1] |
| Cretoxyrhina mantelli | [1] |
[1] Information sourced from Amalfitano et al (2019).
[2] Information sourced from Diedrich (2014).
---
Overview from Fossil Crates
-----
Fossil record
Members of the genus Cretoxyrhina including Cretoxyrhina mantelli are among the best preserved sharks in the world.
Figure 4 from Diedrich (2014) [2] reference:
 [2]
[2]CAPTION: Interpretative drawings of Late Cretaceous Isurus-skeletons. (a) Partly disarticulated skeleton without skull from the late Turonian of Germany (NMB no. 3916 Foerth-Isurus-1); (b) partly disarticulated but nearly complete skeleton from the Niobrara Formation (Coniancian to Campanian) of USA with its last prey, a huge teleostean Xiphactinus audax (in black, redrawn after [18]; KUVP no. 247 after [19]); (c) articulated skeleton from the Niobrara Formation (Coniancian to Campanian) of USA (redrawn after [13]; FHSM no. VP-2187); (d) partly preserved articulated skeleton from the “Senonian” (Coniacian/Santonian) of Italy (redrawn after [20]); (e) partly preserved articulated skeleton from the Niobrara Formation (Coniancian to Campanian) of USA (redrawn after photos of Everhart, STERN no. 01).
NOTE: Diedrich (2014) [2] is/was a proponent of the taxonomic re-assignment of Cretoxyrhina mantelli to Isurus denticulatus much like Glikman (1958) but this re-assignment is in dispute and/or rejected:
The taxonomic status of the genus Cretoxyrhina was discussed by Siverson et al. (2013). The authors rejected Zhelzko's (2000) synonymy of Cretoxyrhina with Pseudoisurus. Glikman (1958) erected the new genus Cretoxyrhina with Oxyrhina mantelli (Agassiz) as type species (Siverson, 1996; Siverson et al., 2013). Later, Glikman (1964) replaced Oxyrhina mantelli with Isurus denticulatus (Glickman, 1957) as type species of the genus Cretoxyrhina without explanation. As implied by Siverson (1996) and Siverson et al. (2013), this represents an invalid taxonomic amendment (see Ride et al., 2000, Art. 68.2). - Amalfitano et al (2019) [1]
The best preserved specimens on record are following:
| Specimen | Excavated from | Reference | Status | No |
| Holotype | Niobrara Chalk in Kansas | Eastman (1984) | Destroyed in World War 2 | [1][2] |
| FHSM VP-2187 | Niobrara Chalk in Kansas | Shimada (1997) | Preserved | [1][2] |
| FHSM VP-323 | Niobrara Chalk in Kansas | Newbery et al (2015) | Preserved | [1] |
| MPPSA-IGVR 36371 | Monte Loffa in Italy | Amalfitano et al (2019) | Preserved | [1] |
| MPPSA-IGVR 45305 | Monte Loffa in Italy | Amalfitano et al (2019) | Preserved | [1] |
---
Largest teeth ever found
| Specimen | Isolated or Associated | Dimensions | Excavated from | Estimated TL | Reference | No |
| FFHM 1972.196 | Isolated | TH = 65mm; CH = 53 mm | Niobrara Chalk in Kansas | Estimated TL = 6.63 m | Everhart (2005); Shimada (2008) | [3][4] |
| MPPSA-IGVR 36371 | Associated | TH = 67 mm; CH = 52 mm | Monte Loffa in Italy | Estimated TL = 6.5 m | Amalfitano et al (2019) | [1] |
Figure S2 in Pimiento et al (2010) [5] for reference:
 [5]
[5]FFHM 1972.196 as depicted in Shimada (2008) [4] for reference:
 [4]
[4]CAPTION: FIGURE 6. Anterior teeth of Cretoxyrhina mantelli from Niobrara Chalk of western Kansas. A, shortest known anterior tooth (FHSM VP- 16522); B, tallest known anterior tooth (FFHM 1972.196; tooth tip re-stored). Left = labial view; right = lingual view.
FFHM 1972.196 in restored form for reference:
 [6]
[6]
---
Largest vertebral centrum ever found
The largest vertebral centrum preserved is of the Cretoxyrhina mantelli specimen MPPSA-IGVR 36371; it has a diameter of 107 mm (Amalfitano et al., 2019) [1]; not shown in the photo below.
 [1]
[1]CAPTION: Fig. 15. Cretoxyrhina mantelli (Agassiz, 1835). Count of incremental bands of individuals MPPSA-IGVR 36371 and 45305. A. Vertebral centrum from MPPSA-IGVR 36371. The black dot indicates the vertebral fulcrum, the black stars indicate the incremental bands (26). B. Vertebral centrum from MPPSA-IGVR 45305. The black dot indicates the vertebral fulcrum, the black stars indicate the incremental bands (21). Scale bars = 10 mm [1,5-column width].
---
TL estimations
Amalfitano et al (2019) [1] provide an overview:
The specimens described herein, especially MPPSA-IGVR 36371 and MPPSA-IGVR 45305 provide some significant data for the estimation of their total length (TL) and longevity. Using the equations of Shimada (2008) on the specimen MPPSA-IGVR 36371, that has a maximum crown height measured on the labial side (EH in Shimada, 2008) of 52 mm and a maximum vertebral diameter (CD in Shimada, 2008) of 107 mm, results in estimated TLs of 650 cm and of 615 cm, respectively. These estimated TLs suggest that MPPSA-IGVR 36371 represents one of the largest individuals of Cretoxyrhina mantelli ever found. The size is comparable to the asymptotic (= maximum) length for Cretoxyrhina mantelli (691 cm) proposed by Shimada (2008), and to the estimated range (640 - 700 cm) for the largest individual described to date (represented by a well-preserved caudal fin; Shimada et al., 2006). MPPSA-IGVR 45305 (42 mm of maximum EH and 98 mm of maximum CD) has an estimated total length of 525 cm based on EH, and 563 cm based on CD. Other relevant specimens are MPPSA-IGVR 45344 - 45345 and MGC-IGVR 81375 - 81376, with the maximum EH of 46 and 47 mm corresponding to TLs of 575 cm and 587.5 cm, respectively. The other specimens exhibit a comparatively smaller size or are more fragmentary and do not include the largest precaudal vertebra or the highest tooth, and for this reason these are not used for size estimates. - Amalfitano et al (2019) [1]
-----
Physiology and anatomy
 [7]
[7]Description: 25-foot Cretaceous era shark Cretoxyrhina. McWane Science Center, Birmingham, AL. Photo credit: John Gnida.
---
Dentition and jaw structure
 [8]
[8]NOTE: This partially preserved dentition is in possession of Dr. Gordon Hubbell (private collection).
---
Partially preserved dentition (specimen FHSM VP-14004) in Bourdon and Everhart (2011) [9] for reference:
 [9]
[9]CAPTION: FACING PAGE - Figure 6, Cretoxyrhina mantelli, FSHM VP-14004 tooth set and examples, individually scaled by group A-F, Tooth set, 3-perspectives, similarly scaled. Scale bar = 3 cm; A, Upper files, basal view; B, Upper files, labial view; C, Lower files labial view; D, Lower files, basal view; E, Upper files, lateral view; F, Lower files, lateral view;
---
Dental pattern in Bourdon and Everhart (2011) [9] for reference:
 [9]
[9]CAPTION: Figure 2, Cretoxyrhina tooth set (FHSM VP-2187) with Comparative Position notations. Tooth set source Shimada (1994), reproduced with permission.
A closer look at specimen FHSM VP-2187 for reference:

 [6]
[6]
A closer look at the skull of specimen FHSM VP-2187 for reference:
 [10]
[10]Reconstructed skull in Shimada (1997) [11] for reference:
 [11]
[11]Head Region - The examination of scales in Cretoxyrhina mantelli suggests that the shark had a conical head. The rostrum does not seem to extend forward greatly from the anterior margin of the relatively wide neurocranium. Therefore, the snout of C. mantelli was probably blunt (Fig. 11). - Shimada (1997) [11]
A closer look at the skull of 25-foot Cretaceous era shark Cretoxyrhina in McWane Science Center, Birmingham, AL:
 [12]
[12]---
Body structure
Figure 1 in Shimada (2008) [4] for reference:
 [4]
[4]CAPTION: FIGURE 1. Late Cretaceous lamniform shark, Cretoxyrhina mantelli. A, nearly complete skeleton, FHSM VP-2187, from Niobrara Chalk, Ellis County, Kansas (see Shimada, 1997c; vertebra immediately below asterisk is vertebra illustrated in Figure 2); B, skeletal reconstruction based on multiple skeletal remains (after Shimada, Cumbaa et al., 2006: fig. 8; maximum total length = approximately 7 m in total length; solid lines = known parts; broken lines = inferential parts).
The present fossil record of Cretoxyrhina mantelli does not allow confirmation of other details seen in the extant lamniforms, such as the presence of spiracles and poorly calcified elements (e.g., ribs and branchial rays), and exact morphology of most fins. However, the present data, at least, suggest that C. mantelli had a conical head with a blunt snout and large eyes (Fig . 11) and a body size similar to extant Carcharodon carcharias (for biology of C. carcharias, see Compagno, 1984). Furthermore, it is possible to suppose from the present data that the body form of Cretoxyrhina mantelli was similar to extant Carcharodon carcharias. - Shimada (1997) [11]
---
Fin structure
Partially preserved pectoral fin of specimen KUVP 49490 in Shimada (2008) [4] for reference:
 [4]
[4]CAPTION: FIGURE 7. Pectoral fin radials (KUVP 49490). with an outline of the entire fin. of Cretoxyrhina(?) (anterior to the top).
Partially preserved caudal fin of specimen CMN 40906 in Shimada et al (2006) [13] for reference:
 [13]
[13] CAPTION: FIGURE 4. Close-up view of putative caudal fin base, showing connection between vertebral column and hypochordal rays in CMN 40906 (scale bar = 10 cm; cf. Figs. 1,2).
---
Regional endothermy
Ferrón (2017) [14] present a well-researched case for regional endothermy in Cretoxyrhinids; see REFERENCES for access to full article.
Figure 4 in Ferrón (2017) [14] for reference:
CAPTION: Fig 4.
(A) Comparison between net cost of swimming (NCS) and routine metabolic rate (RMR) of Cretoxyrhina mantelli at five different temperature scenarios (5°C, 10°C, 15°C, 20°C and 25°C) assuming ectothermy (green) and regional endothermy (pink). Green and pink gradations represent the NCS at different swimming speeds (see color code chart). Note that NCS are constant in all temperature scenarios (see text). RMR estimate is represented with associated 95% individual prediction intervals (in black). (B) Validation test performed in 17 living sharks, including ectothermic taxa (green background) and regional endothermic taxa (pink background), from simultaneous records of their cruise swimming speeds, water temperatures and body masses (data taken from [85]). Inferred RMR are denoted as green and pink intervals for the ectothermic and regional endothermic scenario respectively; Inferred NCS are represented by black dots.
LINK: doi.org/10.1371/journal.pone.0185185.g004
Metabolic ceilings have been proposed several times as constraining the distribution of plants and animals, accounting for their observed latitudinal and altitudinal limits [142–144]. Differences in thermal physiology between ectotherms and endotherms affect their global distributions differently, with ambient temperature being the main constrain for ectothermic organisms [145–147]. Accordingly, this work suggests differences in the potential habitable temperature range of Cretoxyrhina depending on its thermoregulatory capabilities. When considering Cretoxyrhina as an ectothermic shark the model predicts that its energy expenditure of locomotion would not be sustainable over time in waters below 20°C (note in Fig 4A that NCS exceeds RMR below this temperature in the ectothermic scenario). In contrast, such costs would be probably sustainable long-term by a regional endothermic shark in a wider range of water temperatures (RMR could exceed NCS at all the considered temperatures in the regional endothermic scenario, Fig 4A). - Ferrón (2017) [14]
---
Speed and swimming behavior
Cretoxyrhina mantelli was a fast-moving shark with estimated burst swimming capacity of 30.55 km/h.
Figure 2 in Ferrón (2017) [14] for reference:
CAPTION: Fig 2. Scaling of swimming speed in extant fishes and swimming speed inferences in cretoxyrhinids and otodontids.
(A) Cruise and (B) burst relative swimming speeds (U, body lengths*s-1) against body lengths (meters) of living ectothermic and regional endothermic fishes. Adjusted regression lines are showed with associated 95% confidence intervals. (C) Cruise and (D) burst swimming speed estimates (V, km*h-1) of cretoxyrhinids and otodontids, considering them as ectothermic sharks (green) or regional endothermic sharks (pink), with associated 95% individual prediction intervals. Values of absolute (V, km*h-1) and relative (U, BL*s-1) speed estimates are also shown for each case. ** indicates significance at the 0.05 level.
LINK: doi.org/10.1371/journal.pone.0185185.g002
Pointer # 1 in relation:
Otodontids and cretoxyrhinids are thought to have been active macropredators hunting on relatively big and fast swimming prey. This interpretation is based in functional analyses of their dentitions [1,31], trophic level inferences from isotopic data [104] and direct evidence such as coprolites with fish remains [105] or bite marks, fractures and embedded teeth in fossil cetacean, sirenian and marine reptile bones [6,9,10,106–115]. - Ferrón (2017) [14]
NOTE: see Trophic interactions section below.
Pointer # 2 in relation:
In addition, the squamation pattern and some morphofunctional interpretations of cretoxyrhinids also support an adaptation to fast swimming, more congruent with values estimated for the scenario of regional endothermy. Shimada [122] noted that the body surface of Cretoxyrhina was covered by scales with parallel keels separated by U-shaped grooves where the average interkeel distance was approximately 45 microns (see [43]: fig 5 and [122]: fig 8B). These aspects evidence a clear role in drag reduction and allow the unequivocal assignation of Cretoxyrhina scales to a morphotype that is exclusive to the fastest living species of sharks [123–127]. In the same way, some other evident aspects of the squamation of Cretoxyrhina, such as the high density of scales together with a notable overlapping ([122]: fig. 8A and B) or the presence of scales with crown thinning ([43]: fig. 5A and C), have also been interpreted as an adaptation for enhancing hydrodynamic efficiency in fastest pelagic species ([128] and references therein). A few smooth rounded scales have also been described in Cretoxyrhina but they were probably restricted to the snout and possibly other regions exposed to high abrasive stress (scales of Type A in [122]). Similarly, functional interpretations of caudal fin remains also support the idea of Cretoxyrhia as a fast pelagic hunter. Specimen CMN 40906 exhibits the highest Cobb’s and hypochordal ray angles ever recorded in lamniform sharks (49° and 133° respectively), implying fast swimming capabilities in Cretoxyrhina [50]. Thomson and Simanek [129] noted that Cobb’s angles above 30° are characteristic of fast swimming pelagic sharks typified by the endothermic sharks within the family Lamnidae. In fact, the swimming speed calculated from morphological variables of the caudal fin of Cretoxyrhina (≈70 km*h-1, see S2 Text) is clearly incompatible with those predicted here under the assumption that it was an ectothermic shark (≈7 km*h-1). Finally, some other aspects, like the conical head and the shape and number of vertebra in Cretoxyrhina [122] and the absence of dorso-ventral flattening in Cretolamna vertebra [44], also point towards the existence of fusiform bodies in cretoxyrhinids compatible with an active pelagic lifestyle. - Ferrón (2017) [14]
Traces of scales on specimen FHSM VP-2187 in Shimda (1997) [11] for reference:
 [11]
[11]NOTE: Two related Figures depicted side-by-side for a better view.
Traces of scales on specimen specimen CMN 40906 in Shimada et al (2006) [13] for reference:
 [13]
[13]NOTE: Two related Figures depicted side-by-side for a better view.
The outline of the caudal fin is not preserved in CMN 40906. However, the organization of preserved hypochordal rays with respect to the preserved vertebral column (Fig. 4) offers insights into the tail morphology of Cretoxyrhina mantelli. CMN 40906 shows that the anteriormost and preserved posteriormost hypochordal rays are short, whereas the rays between them are markedly elongate. In addition, the base of the anteriormost hypochordal rays in the specimen occurs immediately anterior to the sharp bend (at an angle of ca. 45o) that is observed in the vertebral column. If one considers the sharp bend at face value as a dorsally directed bend of the vertebral column that constitutes the axis of a tail, this skeletal organization is remarkably similar to that of extant sharks with a high aspect ratio: i.e., a lunate (symmetrical) tail with large dorsal and ventral lobes (Fig. 7A). Thomson and Simanek (1977, table 3) listed Rhincodon Smith (Orectolobiformes), Cetorhinus Blainville, Lamna, Isurus, and Carcharodon (Lamniformes) as best representatives of this type of shark, with the caudal portion of the vertebral column bent at an angle of ca. =30o. The caudal fin of other extant sharks is strongly heterocercal: i.e., an asymmetrical tail with a much smaller ventral lobe compared to the dorsal one (Thomson and Simanek, 1977). In these sharks, the vertebral column shows a weak bend or virtually no bend, and the length of the hypochordal rays is more or less uniform across the tail (Figs. 7B, 7C). Therefore, CMN 40906 supports the hypothesis that Cretoxyrhina mantelli had a lunate tail (Fig. 8). It is noteworthy that, because Rhincodon is not a lamniform (Shirai, 1996), and because C. mantelli does not share an immediate common ancestry with Cetorhinus, Lamna, Isurus, or Carcharodon (Shimada, 2005a), the similarity in caudal fin morphology between Cretoxyrhina mantelli and these extant sharks is interpreted to be a homoplasy due to convergent evolution. However, we note that the caudal fin of C. mantelli possibly had a wider angle between the upper and lower lobes and a slightly shorter lower lobe (Fig. 8) compared to the caudal fin of those extant sharks (e.g., Fig. 7A), because the hypochordal rays in CMN 40906 are largely directed ventrally (Figs. 2, 4; as opposed to ventroposteriorly in those extant sharks). We also note that, because the posteriorly located hypochordal rays appear to be thinner and shorter as compared to those in Rhincodon, Cetorhinus, Lamna, Isurus, or Carcharodon, the lobes of the lunate tail in Cretoxyrhina mantelli could have been slightly narrower than the lobes in those extant sharks.
Thomson and Simanek (1977) found that extant sharks with a heterocercal (>= “vertebral bent”) angle of ca. >=30o, not only have a well-developed ventral hypochordal lobe, but also a deep fusiform body with a conical head and a caudal peduncle bearing the lateral fluke. Thomson and Simanek (1977) characterized such sharks as “fast swimming pelagic sharks,” typified by extant lamnids, such as the mako and white sharks. The vertebral bend in CMN 40906 is about 45o, which approximates the maximum possible angle observed in extant sharks (see Thomson, 1976). Therefore, CMN 40906 further strengthens the idea (Shimada, 1997b) that the body form of Cretoxyrhina mantelli (Fig. 8) resembled that of extant lamnids, including the white shark, Carcharodon carcharias. We assume that Cretoxyrhina mantelli also had a caudal peduncle with a lateral fluke. - Shimada et al (2006) [13]
Thomson and Simanek (1977) found that extant sharks with a heterocercal (>= “vertebral bent”) angle of ca. >=30o, not only have a well-developed ventral hypochordal lobe, but also a deep fusiform body with a conical head and a caudal peduncle bearing the lateral fluke. Thomson and Simanek (1977) characterized such sharks as “fast swimming pelagic sharks,” typified by extant lamnids, such as the mako and white sharks. The vertebral bend in CMN 40906 is about 45o, which approximates the maximum possible angle observed in extant sharks (see Thomson, 1976). Therefore, CMN 40906 further strengthens the idea (Shimada, 1997b) that the body form of Cretoxyrhina mantelli (Fig. 8) resembled that of extant lamnids, including the white shark, Carcharodon carcharias. We assume that Cretoxyrhina mantelli also had a caudal peduncle with a lateral fluke. - Shimada et al (2006) [13]
Amalfitano et al (2019) [1] provide further insight in relation:
Swimming behavior and paleoecology Newbrey et al. (2015) observed that the vertebral centra of Cretoxyrhina mantelli are relatively antero-posteriorly compressed when compared to those of other fossil and extant lamniform sharks. The short vertebral centra and the high vertebral count led Newbrey et al. (2015) to hypothesize a carangiform swimming mode for Cretoxyrhina mantelli, implying that it was a moderately fast swimmer with high maneuverability.
The swimming capabilities of sharks can be estimated also through the morphological analysis of the placoid scales (e.g., Reif, 1985). Scale morphology suggests that Cretoxyrhina mantelli was a fast swimming shark (Shimada, 1997d). According to Reif and Dinkelacker (1982), keels (= ridges or riblets) and grooves on the scales that run approximately parallel to the body axis, as observed in Cretoxyrhina mantelli, are characteristic of fast swimming sharks (see also Shimada, 1997d). Reif (1985) recognized six ecological groups of sharks based on their locomotory habits, in which placoid scales have different functions that correlate to the ecology of the various shark species. Only in two groups, the fast swimming pelagic sharks and the large near-shore predators/moderate speed pelagic predators, the morphology of the placoid scales shows an evident hydrodynamic function. Fast swimming pelagic sharks have flat, usually overlapping scale crowns that form a dense pavement (Reif, 1985). Crowns exhibit a rounded posterior end or short cusps. The surface of the crown in this group of sharks is ornamented with fine parallel ridges that have average distances between 40 and 80 mm with U-shaped grooves separating the ridges. The placoid scales of Cretoxyrhina mantelli show all of these features. When found articulated, such as those figured in Shimada (1997d: fig. 8), they have a pavement-like arrangement to reduce hydrodynamic drag. As far as the scale ornamentation is concerned, placoid scales from MPPSA-IGVR 36371 and MGC-IGVR 81375 exhibit a pattern of ridges and grooves nearly identical to that observed by Shimada (1997d), Shimada et al. (2006) and Diedrich (2014) in Cretoxyrhina mantelli from other localities. The distance between the ridges in the specimens described herein ranges between 33 mm and 60 mm, with the mean value falling within the range of fast swimming sharks (Reif, 1985). Moreover, we employed a method utilized by Reif (1985) that was recently used for other fossil sharks (e.g., Marrama et al., 2018), which takes into account the ridge spacing and the scale width. We plotted the average ridge spacing and average crown width from a few individuals (FHSM VP-2187, MPPSA-IGVR 36371, MGC-IGVR 81375; see Tab. A.3) and compared the results with those of fast pelagic hunting sharks and large nearshore hunters/pelagic predators of moderate speed (see Reif, 1985). The results are shown in Fig. 16, in which Cretoxyrhina mantelli clearly falls within the fast pelagic hunting shark group, in particular the ridge spacing is very similar to that of Isurus oxyrhinchus and Sphyrna tudes. The average crown width, however, differs from those observed in these two taxa, but it is more similar to that of Lamna nasus. Comparing the morphology and arrangement of placoid scales of Cretoxyrhina mantelli and those of extant lamniform sharks, the scales of Cretoxyrhina mantelli (see Shimada,1997d: fig. 8) nevertheless differ from those of Isurus oxyrhinchus (see Reif, 1985: pl. 20, 21), which is a very fast hunter adapted to chasing fast moving fishes, such as swordfishes and tunas, in that the placoid scales have a looser arrangement. Actually, the placoid scales arrangement in C. mantelli is more similar to that in Carcharodon carcharias (see Reif, 1985: pl. 23). However, the number of ridges in the scales of C. mantelli (up to nine; Shimada, 1997d) is greater than that observed in I. oxyrhinchus (up to five ridges; Reif, 1985) and Carcharodon carcharias (up to three ridges). Reif (1985) hypothesized that new ridges were added with the expansion of the crown width to maintain constant the distance between the ridges.
Considering all the aspects discussed herein, the conclusions drawn by other authors about caudal fin morphology and metabolic rate estimates (e.g., Kim et al., 2013; Ferron, 2017), and the fossil record of predation attributed to C. mantelli (see Shimada, 1997b; Shimada, Hooks III, 2004; Hone et al., 2018), this Cretaceous lamniform shark was likely a fast swimmer with an ecology in some ways similar to that of the living Carcharodon carcharias (see Shimada, 1997d). - Amalfitano et al (2019) [1]
The swimming capabilities of sharks can be estimated also through the morphological analysis of the placoid scales (e.g., Reif, 1985). Scale morphology suggests that Cretoxyrhina mantelli was a fast swimming shark (Shimada, 1997d). According to Reif and Dinkelacker (1982), keels (= ridges or riblets) and grooves on the scales that run approximately parallel to the body axis, as observed in Cretoxyrhina mantelli, are characteristic of fast swimming sharks (see also Shimada, 1997d). Reif (1985) recognized six ecological groups of sharks based on their locomotory habits, in which placoid scales have different functions that correlate to the ecology of the various shark species. Only in two groups, the fast swimming pelagic sharks and the large near-shore predators/moderate speed pelagic predators, the morphology of the placoid scales shows an evident hydrodynamic function. Fast swimming pelagic sharks have flat, usually overlapping scale crowns that form a dense pavement (Reif, 1985). Crowns exhibit a rounded posterior end or short cusps. The surface of the crown in this group of sharks is ornamented with fine parallel ridges that have average distances between 40 and 80 mm with U-shaped grooves separating the ridges. The placoid scales of Cretoxyrhina mantelli show all of these features. When found articulated, such as those figured in Shimada (1997d: fig. 8), they have a pavement-like arrangement to reduce hydrodynamic drag. As far as the scale ornamentation is concerned, placoid scales from MPPSA-IGVR 36371 and MGC-IGVR 81375 exhibit a pattern of ridges and grooves nearly identical to that observed by Shimada (1997d), Shimada et al. (2006) and Diedrich (2014) in Cretoxyrhina mantelli from other localities. The distance between the ridges in the specimens described herein ranges between 33 mm and 60 mm, with the mean value falling within the range of fast swimming sharks (Reif, 1985). Moreover, we employed a method utilized by Reif (1985) that was recently used for other fossil sharks (e.g., Marrama et al., 2018), which takes into account the ridge spacing and the scale width. We plotted the average ridge spacing and average crown width from a few individuals (FHSM VP-2187, MPPSA-IGVR 36371, MGC-IGVR 81375; see Tab. A.3) and compared the results with those of fast pelagic hunting sharks and large nearshore hunters/pelagic predators of moderate speed (see Reif, 1985). The results are shown in Fig. 16, in which Cretoxyrhina mantelli clearly falls within the fast pelagic hunting shark group, in particular the ridge spacing is very similar to that of Isurus oxyrhinchus and Sphyrna tudes. The average crown width, however, differs from those observed in these two taxa, but it is more similar to that of Lamna nasus. Comparing the morphology and arrangement of placoid scales of Cretoxyrhina mantelli and those of extant lamniform sharks, the scales of Cretoxyrhina mantelli (see Shimada,1997d: fig. 8) nevertheless differ from those of Isurus oxyrhinchus (see Reif, 1985: pl. 20, 21), which is a very fast hunter adapted to chasing fast moving fishes, such as swordfishes and tunas, in that the placoid scales have a looser arrangement. Actually, the placoid scales arrangement in C. mantelli is more similar to that in Carcharodon carcharias (see Reif, 1985: pl. 23). However, the number of ridges in the scales of C. mantelli (up to nine; Shimada, 1997d) is greater than that observed in I. oxyrhinchus (up to five ridges; Reif, 1985) and Carcharodon carcharias (up to three ridges). Reif (1985) hypothesized that new ridges were added with the expansion of the crown width to maintain constant the distance between the ridges.
Considering all the aspects discussed herein, the conclusions drawn by other authors about caudal fin morphology and metabolic rate estimates (e.g., Kim et al., 2013; Ferron, 2017), and the fossil record of predation attributed to C. mantelli (see Shimada, 1997b; Shimada, Hooks III, 2004; Hone et al., 2018), this Cretaceous lamniform shark was likely a fast swimmer with an ecology in some ways similar to that of the living Carcharodon carcharias (see Shimada, 1997d). - Amalfitano et al (2019) [1]
---
Longevity
Cretoxyrhina mantelli experienced intense competitive pressures from other contemporaneous macrophagous taxons (see Overview from Fossil Crates section above), and chances of mortality were high in the neonate stage in particular (Shimada, 2008) [4].
| Specimen | Age | Reference | No |
| FHSM VP-2187 | 15 years old when died | Shimada (2008) | [4] |
| MPPSA-IGVR 45305 | 21 years old when died | Amalfitano et al (2019) | [1] |
| MPPSA-IGVR 36371 | 26 years old when died | Amalfitano et al (2019) | [1] |
Shimada (2008) [4] asserted that Cretoxyrhina mantelli could exceed 30 years in life:
 [4]
[4]- but this inference is not independently cross-validated in the present.
Cretoxyrhina mantelli had estimated TL of 1.27 m at birth (Shimada. 2008) [4]; give or take:
 [4]
[4]Figure 2 in Shimada (2008) [4] for reference:
 [4]
[4]CAPTION: FIGURE 2. Vertebral centrum of Cretoxyrhina mantelli (FHSM VP- 2187) showing diagenetically stained zones interpreted to be growth increment bands on cross-sectional surface of corpus calcareum (after Shimada, 1997a: fig. 1). In this illustration, band count is 15, not including band formed presumably at birth (i.e., band number 0). Arrow points the center of vertebra.
-----
Trophic interactions
Competitive pressures notwithstanding, Cretoxyrhina mantelli was built to kill:
C. mantelli was the largest shark in the Western Interior Sea, reaching lengths of 6+ m (Shimada, 1997a; Shimada et al., 2004). It had razor–sharp teeth as large as 6.5 cm (FFHM 1972.196; per. obs.), and a bite that was powerful enough to shear through 5 cm diameter mosasaur vertebrae (Everhart, 1999, 2004b). - Everhart (2005) [3]
Shimada (1997) [15] highlighted the possibility of Cretoxyrhina mantelli being the apex predator of its time:
Several specimens of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli (Agassiz), from the Niobrara Chalk of Kansas suggest that the shark fed on teleosts, mosasaurs, and possibly plesiosaurs. These animals are active vertebrates, so C. mantelli probably occupied the apex of the food chain in the Late Cretaceous seas. This top predator, however, was probably scavenged frequently by anacoracid sharks. Carcharodon carcharias (great white shark) and carcharhinid sharks are considered as the modern guild counterparts for Cretoxyrhina mantelli and anacoracids, respectively. - Shimada (1997) [15]
There is ample fossil record of trophic interaction(s) of prehistoric sharks [3][15]. Examples of stomach contents and/or cases of trophic interactions of Cretoxyrhina mantelli are presented below:
| Cretoxyrhina mantelli specimen | Prey item | Prey item type | Total Length (TL) | Prey item observations | No |
| KUVP 55060 | Ichthyodectidae indet. | Bony fish | ? | Consumed: smaller than the prey item consumed by KUVP 247. | [15] |
| One tooth of a shark (estimated TL = 3.1 m) in association with remains of the subject. | Xiphactinus audax (ESU 1047)**** | Bony fish | 3 m | Trophic interaction: skull of the prey item found to be bitten. | [16] |
| KUVP 247 | Xiphactinus audax | Bony fish | 4 m | Consumed: prey item is large (premaxilla length = 98mm; vertebral centrum diameter = 46.5 mm). | [2][15] |
| Cut marks noticed on the remains of the subjecta | Protostega gigas (ALAM PV985.10.2) | Turtle | ? | Trophic interaction: numerous bite marks on internal and external surfaces of the remains of ALAM PV985.10.2 noticed; concentrated on both ends and below the ulnar process. | [17] |
| Cut marks noticed on the remains of the subject | Protostega gigas (FMNH PR58) | Turtle | ~1.5 m | Trophic interaction: exact arrangement of the remains of FMNH PR58 are uncertain but it was possible to tell that the shark bit this turtle at least twice from two different angles (e.g., once laterally and another posterolaterally). The prey item had a carapace length of approximately 105 cm. | [17] |
| Fragments of a tooth of a shark (estimated TL = 5 m) in association with remains of the subject | Protostega gigas (FMNH P27452) | Turtle | ~1.5 m | Trophic interaction: exact arrangement of the remains of the remains of FMNH P27452 (the left humerus and left hyoplastron) coupled with distribution of cut marks and their curvature suggest that the shark intercepted this turtle more or less from the front and bit at least twice. The prey item had a carapace length of approximately 105 cm. | [17] |
| KUVP 69102 (TL = 3 m) | Mosasauridae indet. | Mosasaur | ? | Consumed: one Mosasaur vertebra found to be ingested (extensive etching by gastric acid). | [15][18] |
| One tooth of a shark (estimated TL =< 3 m) in association with remains of the subjectb | Platecarpus ? (KUVP 1094)c | Mosasaur | 6 m | Trophic interaction (failure): direct evidence of predation effort on a Mosasaur; subsequent healing and survival of the prey item but post-trauma infection is apparent. | [6][15][18] |
| Two teeth of a shark in association with remains of the subject with cut marks in the mixd | Platecarpus ? (FHSM VP-13283) | Mosasaur | 7 m | Trophic interaction: remains of the prey item clearly shows a bite direction from the right side of the mosasaur's body, indicated by the labial-lingual directions of the embedded Cretoxyrhina teeth and tooth marks. | [15] |
| Several teeth of a shark in association with remains of the subject | Tylosaurus sp. (M.J. and P.A. Everhart collection)e | Mosasaur | 6 - 7 mf | Trophic interaction: skull of the prey item found to be bitten (skull length = 36 inch (914.4 mm) long). | [6][15] |
| Several teeth of a shark in association with remains of the subject | Tylosaurus sp. (FHSM VP–13742)e | Mosasaur | 7 mf | Trophic interaction: skull of the prey item found to be bitten (lower jaw length = 39 inch (990.6 mm) long). | [3][19] |
| Cut marks noticed on the remains of the subject (estimated TL = 5 m) | Elasmosauridae indet. (FFHM 1974.823) | Elasmosaurid | 6 m | Trophic interaction: left front paddle found to be bitten | [3] |
| KUVP 68979 (estimated TL = 6 m) | Elasmosauridae indet. | Elasmosaurid | ? | Consumed: blackish polished pebbles (or gastroliths) of the prey item noticed (3 - 71 mm in respective lengths); over 124 in count. | [3][15] |
a misidentified as Squalicorax in Schwimmer et al (1997); revisited in (Shimada & HOOKS III., 2004) [17]
b misidentified as Squalicorax in Martin and Rothschild (1989); revisited in (Shimada., 1997) [15]; revisit acknowledged in (Rothschild et al., 2005) [18]
c this specimen could be a member of genus Tylosaurus in life but correct identification is not possible due to condition of the remains (Rothschild et al., 2005) [18]
d these teeth are identified as FHSM VP-13284 and FHSM VP-13285 respectively (Shimada., 1997) [15]
e this specimen show signs of recovery [6], but this inference is disputed in (Shimada, 1997) [15]; attack on the skull or head region is typically indicative of predation effort and/or aggressive trophic interaction (Everhart, 2008; Voss et al., 2019; Bastiaans et al., 2020) [20][21][22]
f estimate(s) based on relevant information in Everhart (2002) [23]
---
Rothschild et al (2005) [18] disclose that the angle of approach towards Mosasaurs varied:
The attacks described here were directed from above the mosasaur and the shark and mosasaur were meeting each other, rather than the shark pursuing from behind. (Of course, if the mosasaur was ascending from a dive while it was bitten, the attack would have been directed from the "back'). This suggests that the mosasaur was swimming at some depth before the encounter and perhaps met the shark while surfacing. In at least one case (KUVP 1094), the angle of the shark attack is strongly inclined to the mosasaur vertebral column. - Rothschild et al (2005) [18]
Following image is helpful in this regard:
 [6]
[6]Related information in the following link of [6]: oceansofkansas.com/bite.html
Rothschild et al (2005) [18] noted that Cretoxyrhina mantelli was bigger than any mosasaur at TL parity:
Except for other mosasaurs, the only animals in the Cretaceous seas large enough to attack adult mosasaurs were probably sharks. Although most Cretaceous sharks did not grow beyond lengths of about three metres, one species, Cretoxyrhina mantelli, measured about five metres in length when adult; very large individuals probably reached over six metres (Shimada, 1997). A recent discovery (FHSM VP-14010) certainly documents that Cretoxyrhina mantelli reached lengths of at least 5.5 m (Corrado et al., 2003). It should be noted here, too, that a 5-metre shark would have been considerably heavier than a (much more slender) 5-metre mosasaur. - Rothschild et al (2005) [18]
---
Summary of Shimada (1997) [15] for reference:
 [15]
[15]Rothschild et al (2005) [18] noticed and examined several cases of trophic interactions of prehistoric sharks with Mosasaurs and asserted that the survival rate of Mosasaurs was much better in trophic interactions with sharks in the (TL = 2 - 3 m) range but attacks from much larger sharks were most likely FATAL:
What can we learn from shark injuries on mosasaurs? The survived injuries described here are from sharks between two and three metres in length. Attacks from larger sharks (if any) were probably fatal, e.g. in FHSM VP-13283, figured by Shimada (1997, fig. 4), that includes five vertebrae severed from the middle of the back of a 7-metre mosasaur. The mosasaurs that survived bite injuries are not large, ranging in length from five to seven metres. All of these survived bites are on the tail. It seems likely that attacks including bites elsewhere were normally fatal. Survival of the mosasaur suggests that it successfully defended itself. Sharks may have geared their attacks to moments when the mosasaur was more vulnerable.
A 6-metre Cretoxyrhina in the University of Kansas collections (KUVP 68979) has >124 gastroliths associated with it (Everhart, 2000). These could only have come from ingestion of part of a long-necked plesiosaur (elasmosaurid) and provides evidence that sharks also fed on those huge animals. Shimada & Hooks (2004) reported Cretoxyrhina bites on large marine protostegid turtles. While those examples do not permit distinguishing between an attack on a live individual and carcass scavenging, it does provide additional evidence to support the suggestion that Cretoxyrhina ate large marine reptiles. - Rothschild et al (2005) [18]
A 6-metre Cretoxyrhina in the University of Kansas collections (KUVP 68979) has >124 gastroliths associated with it (Everhart, 2000). These could only have come from ingestion of part of a long-necked plesiosaur (elasmosaurid) and provides evidence that sharks also fed on those huge animals. Shimada & Hooks (2004) reported Cretoxyrhina bites on large marine protostegid turtles. While those examples do not permit distinguishing between an attack on a live individual and carcass scavenging, it does provide additional evidence to support the suggestion that Cretoxyrhina ate large marine reptiles. - Rothschild et al (2005) [18]
NOTE: Mosasaurs approaching 7 m in TL are much larger in comparison to those around 5 m in TL as apparent in Figure 6 of Everhart (2008) [20] below:
 [20]
[20]CAPTION: Figure 6. Relative sizes of the two mosasaurs (7 m and 5 m). Scale = 1 m. (Adapted from Williston, 1898)
Everhart (2008) [20] disclosed a case of trophic interaction between a Mosasaur (TL = 7 m) and another Mosasaur (TL = 5 m) in fact; the larger Mosasaur aimed for the skull of the other Mosasaur and crushed it. Mosasaurs exceeding 5 m in TL were adults of some species (Everhart, 2002; Everhart, 2008) [23][20].
Additional evidence of trophic interactions with Mosasaurs
Related information in following link of [6]: oceansofkansas.com/mosapath.html
There is additional evidence of the fact that Mosasaurs were VULNERABLE to trophic interactions with contemporaneous sharks. This dynamic is most apparent in trophic interaction(s) of the Tylosaurus specimen FHSM VP-13750 with two sharks at different points in time.
Everhart (1999) discussed FHSM VVP-13750, a 'twice-bitten' distal end of the tail of a mosasaur from the Smoky Hill Chalk. It consists of 25 caudal vertebrae and a fused distal segment that includes at least five vertebrae (total length about 60 cm). All of the vertebrae are partially digested and some appear to have been bitten through (the result of several bites by the predator, most likely a large shark). The specimen represents an earlier attack that probably removed the tip of the mosasaur's tail, resulting in an infection that fused the five posteriormost remaining vertebrae, which healed over a fairly long period of time (similar to KUVP 1094), and then suffered another attack (equally likely to have been the result of a fatal encounter with a shark, or scavenging). Everhart (1999) described another Platecarpus specimen from the KU collections (KUVP 4862), which preserves unhealed Cretoxyrhina bite marks on the skull and dorsal vertebrae. - Rothschild et al (2005) [18]
+
Most of the occurrences of fused vertebrae appear to be attributable to shark attacks. Large and relatively slow-moving mosasaurs seem to have been a popular target for Cretaceous sharks. FHSM VP-13750 certainly exemplifies this propensity. - Rothschild and Everhart (2015) [24]
Tylosaurus specimen FHSM VP-13750 contain a shark tooth driven into it:
 [24]
[24]CAPTION: Fused caudal vertebrae at the end of the tail of a “club tail” Tylosaurus (FHSM VP-13750) in left lateral view (upper) and right lateral view (lower). Anterior is to the left. The embedded tooth of a shark, the apparent cause of the infection, is located within the white oval. After healing, the fused mass and 28 associated caudal vertebrae of this specimen were subsequently consumed and partially digested by another predator. Scale bar = 2 cm.
Closer look:

 [6]
[6]This Mosasaur was a juvenile at the time of the afore-depicted instance of trophic interaction with a shark (trophic interaction # 1) and recovered:
FHSM VP-13750 represents an unusual case where a fused section of 3-4 caudal vertebrae with the remains of an embedded shark tooth (FHSM VP-17985) show that a juvenile Tylosaurus survived an initial shark bite near the end of its tail. The wound became infected and resulted in the fusion of these terminal vertebrae, evidence that the mosasaur survived the initial bite for an extended period of time. - Rothschild and Everhart (2015) [24]
This Mosasaur endured the FIRST attack (trophic interaction # 1) but suffered mobility degradation and could not endure SECOND attack (trophic interaction # 2):
The fusion in FHSM VP-322 is attributed to trauma with subsequent infection, given the multiple puncture wounds and other evidence of infection. FHSM VP-13750 clearly documents a shark attack, infection and healing, then subsequent ingestion, while the fused vertebrae of FHSM VP-17984 simply indicate that the mosasaur survived the initial injury. In all three of these specimens, the less efficient “club tail” may have contributed to the mosasaur’s lessened ability to avoid predators or to acquire food (Martin and Bell 1995), although FHSM VP-322 appears to have been a nearly fully grown adult at the time of death. - Rothschild and Everhart (2015) [24]
The another predator in the case of specimen FHSM VP-13750 is believed to be a shark as well:
Although the FHSM VP-13750 specimen has a “club tail” fusion of the terminal vertebrae, it also includes 28 additional caudal vertebrae, which along with the fused portion, have been partially digested, and later regurgitated, most likely by another shark (Everhart 1999, 2005). - Rothschild and Everhart (2015) [24]
Full view of Tylosaurus specimen FHSM VP-13750 for reference:
 [6]
[6]Description: At first glance, this pile of mosasaur caudal vertebrae (FHSM VP-13750) appeared to be evidence of just another mosasaur tail bitten off by a large shark and partially digested before being regurgitated. However, the odd shaped lump of bone in the lower left turns out to be at least 3 and probably 4 caudal vertebrae that have been fused together as the result of an infection. It appears likely that the mosasaur had the end of it's tail bitten off by an unknown predator (probably a shark). The wound became infected, fusing the vertebrae and more or less forming a bony club at the end of the tail as it healed. The mosasaur survived the initial attack but then was "sliced and diced" some time afterwards by a large shark, most likely Cretoxyrhina [6].
-----
Evolution and timeline of existence
Cretoxyrhina mantelli had a cosmopolitan distribution, and the current impression is that it might be a chronospecies:
The shark specimens from the ʻlastameʼ lithozone of the Scaglia Rossa Formation can be unquestionably referred to the genus Cretoxyrhina, because of the presence of narrow and bladelike crowns. The oblique heels on the side of the cusp, the absence of the lateral cusplets, and the asymmetric, sharp pointed and rather robust crown (if compared to other congeneric species) support the assignment of the specimens to Cretoxyrhina mantelli. Cretoxyrhina mantelli had a cosmopolitan distribution, since it has been reported in Europe, Russia, Africa, North America and Brazil (Cappetta, 2012). The genus Cretoxyrhina includes also the species C. denticulata (Glickman, 1957) and C. agassizensis (Underwood, Cumbaa, 2010), both of which are diagnosed by the presence of lateral cusplets (with sharp apices in C. agassizensis, and rounded apices in C. denticulata) in many of the lateroposterior teeth (see Underwood, Cumbaa, 2010; Newbrey et al., 2015). C. agassizensis teeth also exhibit a slender and generally straight cusp (also in lateroposterior teeth) than C. mantelli and C. denticulata (see Underwood, Cumbaa, 2010), and incomplete cutting edges on small juvenile anterior teeth (Newbrey et al., 2015). The latter character, however, cannot be taken into account to compare and differentiate adult teeth from the other species. Some associated dentitions of C. mantelli exhibit almost upright crown on lateroposterior teeth (e.g., Eastman, 1894), but this condition may be related to gynandric heterodonty (Siverson M., pers. comm.). Two of the three species have partially overlapping stratigraphic distributions, because C. denticulata ranges from the lower Cenomanian to the lower middle Cenomanian, C. agassizensis ranges from the upper middle Cenomanian to lower middle Turonian (Newbrey et al., 2015), whereas Cretoxyrhina mantelli is reported globally from the upper Cenomanian to the Campanian (Bourdon, Everhart, 2011; Cappetta, 2012; Shimada, 1997e). Another species of Cretoxyrhina, the upper Albian-lower Cenomanian C. vraconensis (Zhelezko, 2000), was revised by Siverson et al. (2013) and clearly differs from C. mantelli in having cusplets and different tooth morphologies in corresponding dental positions (for details see Siverson et al., 2013: p. 14). The species of the genus Cretoxyrhina might possibly represent chronospecies of a single evolutionary lineage (see Newbrey et al., 2015). This evolutionary lineage was characterized by the progressive reduction of lateral cusplets and the progressive increasing size and robustness of teeth throughout its temporal range (see Underwood, Cumbaa, 2010; Cook et al., 2013; Siverson, Lindgren, 2005). An increase in tooth size (probably corresponding to an increase in body size; see “Paleobiological Remarks” for individual length estimates), a significant decrease of crown height-crown width ratio and a loss of lateral cusplets are recorded in the upper Cenomanian-Coniacian interval (see Shimada, 1997e; Siverson, Lindgren, 2005) and the size of the teeth of the Italian specimens indicates that the teeth from the middleupper Turonian had already reached a size similar to those of the Coniacian specimens. - Amalfitano et al (2019) [1]
Geological timescale for reference:
 [25]
[25]| Oldest fossils of Cretoxyrhina mantelli | The upper Cenomanian Bonarelli Level | [1] |
| Youngest fossils of Cretoxyrhina mantelli | Specimen TMP 2011.048.0274 from the Late Campanian Bearpaw Formation in Alberta, Canada | [26] |
---
EXTINCTION
Environmental shifts tiggered decline of Cretoxyrhinids in the Western Interior Seaway:
Stewart (1990) suggests that a general cooling of the Western Interior Sea led to its extirpation in North America, while Everhart (2005a) noted that the decline of Cretoxyrhina coincides with the rise of giant marine lizards called mosasaurs (e.g. Tylosaurus proriger) that would have competed with this large shark as an apex predator. Since Cretoxyrhina is also regarded as a pelagic shark (Shimada 1997f), the shallowing and narrowing of the seaway during the regressive phase of the Niobrara cyclothem may have eliminated its preferred environment within the Western Interior Sea, but that does not explain its worldwide extinction later in the Campanian. - Bourden and Everhart (2011) [27]
- although this development is not sufficient to explain extinction of Cretoxyrhinids on a wider level [27].
Rothschild et al (2005) [18] suggest that Cretoxyrhina mantelli suffered extinction by the onset of the Late Campanian:
By the onset of the late Campanian, Cretoxyrhina mantelli had become extinct (Stewart, 1990; but see also Siverson, 1992), leaving the mosasaurs behind as the dominant marine predators. An additional survey of the Natuurhistorisch Museum Maastricht collections, which houses much younger (mainly Maastrichtian and some late Campanian) mosasaur remains, did not yield any evidence of healed shark bite marks. The largest sharks known from the Maastricht seas were Squalicorax, which reached an estimated maximum length of about three metres, and the similar-sized or perhaps slightly larger Cretalamna appendiculata. Although absence of evidence is not necessarily evidence of absence, it is tempting to assume that by the end of the Cretaceous, mosasaurs had finally established themselves as the exclusive top predator in the marine ecosystem, having no other animals to fear other than mosasaurs. We are, however, fully aware that this study represents two snapshots in space and time only, so more work on other collections is certainly needed in order to obtain a more complete picture. - Rothschild et al (2005) [18]
Competitive pressures of gigantic Mosasaurs such as genus Tylosaurus are inferred to have adversely impacted Cretoxyrhinids [27], but competitive replacement is more likely in the case of species that are rare and/or geo-graphically restricted (Myers and Lieberman, 2011) [28].
Lindgren (2004) [29] highlight a near-extinction event near the Mid-Campanian:
As pointed out by Lindgren & Siverson (2003, 2004), the stratigraphical ranges of the mosasaur taxa identified from the latest early Campanian–early late Campanian interval in southern Sweden show remarkable similarities to those from coeval strata in the Western Interior and Gulf Coast of North America (see e.g. Russell 1988; Kiernan 2002, fig. 2). By the end of the early Campanian, taxon-rich and seemingly healthy assemblages, dominated by Tylosaurus Marsh, 1872, Halisaurus Marsh, 1869, Platecarpus Cope, 1869 and/or Clidastes (see Nicholls & Russell 1990; Fig. 6 herein), were severely affected by an unknown agent resulting in a radical alteration in both diversity and faunal composition (Russell 1994; Kiernan 2002; Lindgren & Siverson 2004). Within a few hundred thousand years, or even less, mosasaur faunas that had flourished since the late Coniacian (at least in North America) virtually collapsed. Platecarpus and Clidastes disappeared, and Halisaurus vanished from the coastal waters of the southern part of the Fennoscandian Shield, the Western Interior and the Gulf Coast, although the genus persisted on the eastern coast of present-day USA and in western Africa until the end of the Maastrichtian (Lingham-Soliar 1991; Holmes & Sues 2000). Tylosaurus survived a little longer than did Clidastes and Platecarpus, ranging throughout the Bx. balsvikensis zone in southern Sweden and perhaps also through most of the late Campanian in North America (Bullard 2003).
In North America, these primitive ʻNiobrara ageʼ sensu Russell (1988, 1994) mosasaur faunas were replaced (after a period with few mosasaurs present of any kind) by new, often less diverse, ʻNavesinkan ageʼ assemblages dominated by Mosasaurus, Plioplatecarpus, and Prognathodon (Russell 1994; Kiernan 2002). The data presented herein suggest that the composition of the late Campanian and earliest Maastrichtian mosasaur assemblages in Scandinavia is strikingly similar to those in coeval strata of North America (Fig. 6). Although additional high-resolution stratigraphical work is needed, the regional extinctions of Platecarpus and Clidastes probably represent local expressions of a single isochronous event rather than independent events occurring at the same time by coincidence (Lindgren & Siverson 2003, 2004). As stated above, the mechanism behind this profound mid-Campanian reorganization event remains unknown. Russell (1994) suggested that the Manson impact event (in Iowa, USA) caused faunal disruption, which might have benefited the evolution of more advanced forms of mosasaurs (i.e. species derived from survivors of the crisis). However, Izett et al. (1993) demonstrated that the Manson impact structure formed at approximately 74 Ma (i.e. during late Campanian time), and consequently this event cannot have caused the turnover near the early/late Campanian boundary (dated at 80.5 Ma; see Fig. 6 here). Hopefully, future in depth research will uncover the extent and mechanism/s responsible for this highly interesting event in mosasaur evolution.- Lindgren (2004) [29]
In North America, these primitive ʻNiobrara ageʼ sensu Russell (1988, 1994) mosasaur faunas were replaced (after a period with few mosasaurs present of any kind) by new, often less diverse, ʻNavesinkan ageʼ assemblages dominated by Mosasaurus, Plioplatecarpus, and Prognathodon (Russell 1994; Kiernan 2002). The data presented herein suggest that the composition of the late Campanian and earliest Maastrichtian mosasaur assemblages in Scandinavia is strikingly similar to those in coeval strata of North America (Fig. 6). Although additional high-resolution stratigraphical work is needed, the regional extinctions of Platecarpus and Clidastes probably represent local expressions of a single isochronous event rather than independent events occurring at the same time by coincidence (Lindgren & Siverson 2003, 2004). As stated above, the mechanism behind this profound mid-Campanian reorganization event remains unknown. Russell (1994) suggested that the Manson impact event (in Iowa, USA) caused faunal disruption, which might have benefited the evolution of more advanced forms of mosasaurs (i.e. species derived from survivors of the crisis). However, Izett et al. (1993) demonstrated that the Manson impact structure formed at approximately 74 Ma (i.e. during late Campanian time), and consequently this event cannot have caused the turnover near the early/late Campanian boundary (dated at 80.5 Ma; see Fig. 6 here). Hopefully, future in depth research will uncover the extent and mechanism/s responsible for this highly interesting event in mosasaur evolution.- Lindgren (2004) [29]
Ikejiri and Zhang (2020) [30] provide further insight in relation:
Eighty-nine marine vertebrate species, including cartilaginous and bony fish and marine reptiles, from northern Gulf of Mexico – located about 500 km from the Chicxulub crater – offer a unique opportunity to determine an extinction process during the last 20 million years of the Late Cretaceous. Our diversity data show two separate extinction events: (i) the ‘Middle Campanian Crisis’ (about 77 Mya) and (ii) the end-Maastrichtian (66 Mya) events. Whether this stepwise pattern of extinctions occurred locally or globally cannot be determined at present due to the lack of a dataset of the marine vertebrate record for reliable comparison. However, this stepwise pattern including the Middle Campanian and end-Maastrichtian events for, at least, a 13 million-year interval indicates long-term global marine environmental changes (e.g., regression, ocean water chemistry change). Because most Cretaceous marine vertebrates already disappeared in the Gulf of Mexico prior to the latest Maastrichtian, the Chicxulub Impact may not be considered as the most devastating extinction event for the community. - Ikejiri and Zhang (2020) [30]
It is possible that near- and mid- Campanian disruptions created 'sustainability crisis' for Cretoxyrhina mantelli and contributed to its decline and subsequent extinction on a wider level.
-----
REFERENCES
[1] Amalfitano, J., Giusberti, L., Fornaciari, E., Dalla Vecchia, F. M., Luciani, V., Kriwet, J., & Carnevale, G. (2019). Large deadfalls of the ʻginsuʼ shark Cretoxyrhina mantelli (Agassiz, 1835)(Neoselachii, Lamniformes) from the Upper Cretaceous of northeastern Italy. Cretaceous Research, 98, 250-275.
[2] Diedrich, C. G. (2014). Skeleton of the fossil shark Isurus denticulatus from the Turonian (Late Cretaceous) of Germany—ecological coevolution with prey of mackerel sharks. Paleontology Journal, 2014.
[3] Everhart, M. J. (2005). Bite marks on an elasmosaur (Sauropterygia; Plesiosauria) paddle from the Niobrara Chalk (Upper Cretaceous) as probable evidence of feeding by the lamniform shark, Cretoxyrhina mantelli. PalArch, Vertebrate Paleontology, 2(2), 14-24.
[4] Shimada, K. (2008). Ontogenetic parameters and life history strategies of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli, based on vertebral growth increments. Journal of Vertebrate Paleontology, 28(1), 21-33.
[5] Pimiento, C., Ehret, D. J., MacFadden, B. J., & Hubbell, G. (2010). Ancient nursery area for the extinct giant shark Megalodon from the Miocene of Panama. PLoS one, 5(5), e10552.
[6] Link: oceansofkansas.com/KS-sharks.html
[7] Link: allosaurusroar.com/mcwane-science-center-birmingham-al/
[8] Link: tellmewhereonearth.com/Sharks_Page_1.htm
[9] Bourdon, J., & Everhart, M. J. (2011). Analysis of an associated Cretoxyrhina mantelli dentition from the Late Cretaceous (Smoky Hill Chalk, Late Coniacian) of western Kansas. Transactions of the Kansas Academy of Science, 114(2), 15-32.
[10] Link: sternbergca.fhsu.edu/index.php/Detail/objects/74c6eaaa-30cb-4327-9877-5d8611c95aea#
[11] Shimada, K. (1997). Skeletal anatomy of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli from the Niobrara Chalk in Kansas. Journal of Vertebrate Paleontology, 17(4), 642-652.
[12] Link: www.bonhams.com/auctions/17517/lot/37/?category=list
[13] Shimada, K., Cumbaa, S. L., & Rooyen D. V. (2006). Caudal fin skeleton of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli, from the Niobrara Chalk of Kansas. New Mexico Museum of Natural History and Science Bulletin, 35, 185-192.
[14] Ferrón, H. G. (2017). Regional endothermy as a trigger for gigantism in some extinct macropredatory sharks. PLoS one, 12(9), e0185185.
[15] Shimada, K. (1997). Paleoecological relationships of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli (Agassiz). Journal of Paleontology, 926-933.
[16] Shimada, K., & Everhart, M. J. (2004). Shark-bitten Xiphactinus audax (Teleostei: Ichthyodectiformes) from the Niobrara Chalk (Upper Cretaceous) of Kansas. The Mosasaur, 7, 35-39.
[17] Shimada, K., & HOOKS III, G. E. (2004). Shark-bitten protostegid turtles from the Upper Cretaceous Mooreville Chalk, Alabama. Journal of Paleontology, 78(1), 205-210.
[18] Rothschild, B. M., Martin, L. D., & Schulp, A. S. (2005). Sharks eating mosasaurs, dead or alive?. Netherlands Journal of Geosciences, 84(3), 335-340.
[19] Everhart, M. J. (2005). Tylosaurus kansasensis, a new species of tylosaurine (Squamata, Mosasauridae) from the Niobrara Chalk of western Kansas, USA. Netherlands Journal of Geosciences, 84(3), 231-240.
[20] Everhart, M. J. (2008). A bitten skull of Tylosaurus kansasensis (Squamata: Mosasauridae) and a review of mosasaur-on-mosasaur pathology in the fossil record. Transactions of the Kansas Academy of Science, 111(3), 251-262.
[21] Voss, M., Antar, M. S. M., Zalmout, I. S., & Gingerich, P. D. (2019). Stomach contents of the archaeocete Basilosaurus isis: Apex predator in oceans of the late Eocene. PLoS one, 14(1), e0209021.
[22] Bastiaans, D., Kroll, J. J., Cornelissen, D., Jagt, J. W., & Schulp, A. S. (2020). Cranial palaeopathologies in a Late Cretaceous mosasaur from the Netherlands. Cretaceous Research, 112, 104425.
[23] Everhart, M. J. (2002). New data on cranial measurements and body length of the mosasaur, Tylosaurus nepaeolicus (Squamata; Mosasauridae), from the Niobrara Formation of western Kansas. Transactions of the Kansas Academy of Science, 105(1), 33-43.
[24] Rothschild, B., & Everhart, M. J. (2015). Co-ossification of vertebrae in mosasaurs (Squamata, Mosasauridae); Evidence of habitat interactions and susceptibility to bone disease. Transactions of the Kansas Academy of Science, 118(3-4), 265-275.
[25] Adopted from: www.geosociety.org/GSA/Education_Careers/Geologic_Time_Scale/GSA/timescale/home.aspx
[26] Cook, T. D., Brown, E., Ralrick, P. E., & Konishi, T. (2017). A late Campanian euselachian assemblage from the Bearpaw Formation of Alberta, Canada: some notable range extensions. Canadian Journal of Earth Sciences, 54(9), 973-980.
[27] Bourdon, J., & Everhart, M. J. (2011). Analysis of an associated Cretoxyrhina mantelli dentition from the Late Cretaceous (Smoky Hill Chalk, Late Coniacian) of western Kansas. Transactions of the Kansas Academy of Science, 114(2), 15-32.
[28] Myers, C. E., & Lieberman, B. S. (2011). Sharks that pass in the night: using Geographical Information Systems to investigate competition in the Cretaceous Western Interior Seaway. Proceedings of the Royal Society B: Biological Sciences, 278(1706), 681-689.
[29] Lindgren, J. (2004). Stratigraphical distribution of Campanian and Maastrichtian mosasaurs in Sweden–evidence of an intercontinental marine extinction event?. GFF, 126(2), 221-229.
[30] Ikejiri, T., Lu, Y., & Zhang, B. (2020). Two-step extinction of Late Cretaceous marine vertebrates in northern Gulf of Mexico prolonged biodiversity loss prior to the Chicxulub impact. Scientific reports, 10(1), 1-13.














































